Icaritin literature
Synthesis of icariin from kaempferol through regioselective methylation and para-Claisen - Cope rearrangement
Mei, Qinggang,Wang, Chun,Zhao, Zhigang,Yuan, Weicheng,Zhang, Guolin
, p. 1220 - 1225 (2015)
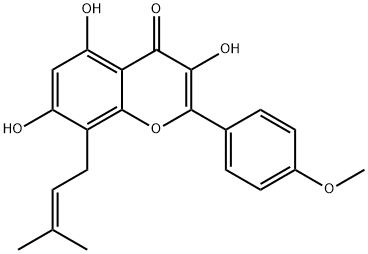
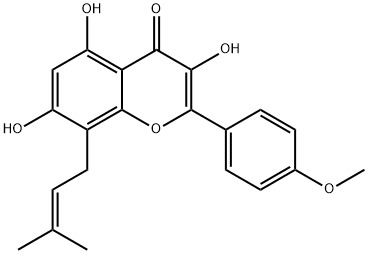
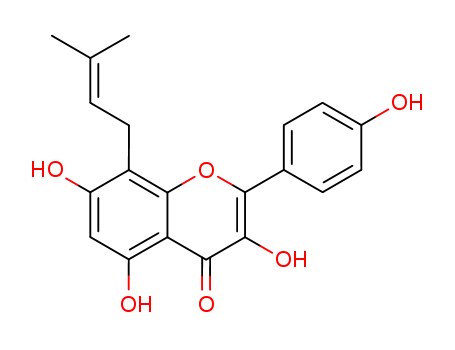
The hemisynthesis of the naturally occurring bioactive flavonoid glycoside icariin (1) has been accomplished in eleven steps with 7% overall yield from kaempferol. The 4?-OH methylation of kaempferol, the 8-prenylation of 3-O-methoxymethyl-4?-O-methyl-5- O-prenyl-7-O-benzylkaempferol (8) via para-Claisen-Cope rearrangement catalyzed by Eu(fod)3 in the presence of NaHCO3 , and the glycosylation of icaritin (3) are the key steps.
A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells
Huang, Xin,Zhu, Danyan,Lou, Yijia
, p. 26 - 36 (2007)
Icariin and icaritin with prenyl group have been demonstrated for their selective estrogen receptor modulating activities. We screened their effects on cell growth in human prostate carcinoma PC-3 cell line (estrogen receptor positive) in vitro. PC-3 cell line was used for the measurement of anti-carcinoma activities of 0-100 μmol/l icaritin and 30 μmol/l icariin. 1 μmol/l 17-β estradiol (E2) served as the estrogen positive control, and 1 μmol/l ICI 182,780 [7 α-[9 (4,4,5,5,5-pentafluoropentyl) sulfinyl] nonyl]-estra-1,3,5(10)-triene-3,17h-diol]] served as the specific estrogen receptor antagonist. Primary cultured rat prostate basal cells used as cell growth selective control. The growth-inhibitory effects were analyzed using MTT assay, and fluorochrome staining, flow cytometry, and immunoblotting were employed to illustrate the possible mechanisms. When treated with icaritin for 24 to 72 h, cell growth was strongly inhibited (at 48 h IC50 was 10.74 ± 1.59 μmol/l, P < 0.001) companied with a mitochondrial transmembrane potential (_Ψm) drop. Meanwhile, few changes in IC50 could be observed when co-incubated with ICI 182,780. Icaritin-induced growth inhibition was associated with G1 arrest (P < 0.05), and G2-M arrest depending upon doses. Consistently with G1 arrest, icaritin increased protein expressions of pRb, p27Kip1 and p16Ink4a, while showed decrease in phosphorylated pRb, Cyclin D1 and CDK4. Comparatively, icariin has much lower effects on PC-3 cells and showed only weak G1 arrest, suggesting a possible structure-activity relationship. These findings suggested a novel anticancer efficacy of icaritin mediated selectively via induction of cell cycle arrest but not associated with estrogen receptors in PC-3 cells.
Magnesium dicarboxylates promote the prenylation of phenolics that is extended to the total synthesis of icaritin
Fu, Xuewen,Lu, Xiaoxia,Wang, Chun,Wen, Yongju,Xiong, Wei,Zhang, Guolin,Zhang, Jichao
, p. 1117 - 1124 (2022/02/16)
The prenylation of phenolic substrates promoted by magnesium dicarboxylates was developed. An investigation of the scope demonstrated that substrates with electron-donating group(s) gave better yields than those with electron-withdrawing group(s). Althoug
Phosphate ester derivative of herba epimedii as well as preparation method and application of phosphate ester derivative
-
Paragraph 0033-0038, (2021/06/26)
The invention belongs to the technical field of medicines, and discloses a phosphate ester derivative of herba epimedii as well as a preparation method and application of the phosphate ester derivative. The chemical structural formula of the phosphate ester derivative of the herba epimedii is represented by the formula (1), wherein R1 is selected from groups defined in the specification, or R2 is selected from H or R1, and R3 is selected from H or a group defined in the specification. According to the phosphate ester derivative of the herba epimedii, the cytotoxicity of the partially modified derivative is remarkably enhanced compared with that of an unmodified derivative, and meanwhile the phosphate ester derivative of the herba epimedii has the anti-osteoporosis effect. The phosphate ester derivative of the herba epimedii can be applied to the field of preparation of drugs for prostate cancer cells, anti-osteoporosis health-care products, pharmaceutical excipients or drugs.
Convenient and efficient total synthesis method for icaritin and derivatives of icaritin
-
, (2020/02/06)
The invention belongs to the field of natural medicine synthesis and particularly relates to a convenient and efficient total synthesis method for icaritin and derivatives of icaritin. The specific technical scheme is as follows: 2'-hydroxyacetophenone and benzaldehyde are used as raw materials, firstly, isopentene groups are introduced in aromatic rings of raw materials under the catalysis of anorganic polyacid metal ion complex, a flavonol framework is constructed under mild and green conditions, and an isopentenyl flavone compound comprising the icaritin and derivatives of icaritin is further synthesized. The method effectively overcomes the limitation of poor substrate solubility and poor regioselectivity when flavone is constructed firstly and then isopentene groups are introduced, the problems of frequent introduction and removal of protecting groups in a conventional isopentenylation method are solved, and the synthesis route is greatly simplified; and meanwhile, the problems of complex products and more byproducts in an isopentene group rearrangement method are solved. The total synthesis method provided by the invention is mild in condition, convenient to operate, high intotal yield and suitable for mass production of the isopentenyl flavone compound.
ANALOGS OF THE NATURAL PRODUCT ICARIIN
-
Paragraph 00168; 00192, (2020/03/02)
Provided herein are analogs of the natural product icariin represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. The analogs can be used to modulate (e.g., inhibit, such as by competitive inhibition) PDE5 and thereby treat a wide range of PDE5- mediated diseases, including cardiovascular, gastrointestinal, pulmonary, musculoskeletal, neurological and reproductive diseases. Also provided herein are compositions and methods including compounds of Structural Formula (I).