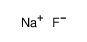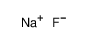Sodium fluoride Appearance/Package/Shipping/Storage
Package
25kg/bag , Double plastic bag inside;
Storage condition
Ventilation, cool place, sun protection, moisture-proof, and rainproof;
Sodium fluoride Application
Used in the paper industry, toothpaste production, chemical reagents, as well as acid washing of steel strips and stainless steel, raw materials for manufacturing other fluorides, adhesives, and paper production.
Sodium fluoride literature
Synthesis of hexagonal NaY(Gd)F4 ultrafine nanocrystals using sodium acetate as ionic mediator
Bart?něk, Vilém,Poryvai, Anna,Ulbrich, Pavel
, p. 142 - 145 (2017)
Hexagonal phase ultrafine nanoparticles of NaY(Gd)F4 were synthesized using chloride precursors and sodium acetate to provide liquid environment for ionic transportand as a source of sodium ions. Prepared ultrafine nanocrystals were analysed by XRD, laser Doppler electrophoresis and TEM measurements. Absence of fluoroacetic acid was confirmed by NMR of wash water obtained by dispersion centrifugation. Synthesized nanocrystals were 8.9?nm in diameter and had a hexagonal crystal structure. The method is appropriate for further experimental development, e.g. the synthesis of luminescent fluorides based on NaY(RE)F4 system, and for possible industrial application because of its ability to prepare large amounts of nanocrystals and due to the fact that the process is affordable and environmental friendly.
Sorption and desorption of BrF3 on NaF: Studies on thermodynamics and kinetics
Zherin,Rudnikov,Ostvald,Sobolev,Amelina
, p. 25 - 33 (2019)
Temperature dependence of bromine trifluoride vapor pressure over its adduct with sodium fluoride has been determined; the adduct normal dissociation temperature has been determined. Kinetics of sorption and desorption processes in BrF3(gas)–NaF(solid) system have been studied. The equation was proposed to illustrate the link between forward reaction rate (Ка) described by Arrhenius equation and experimental value (Ki): Ki= Kа? (Pa/Peq)m, where Ki – observed reaction rate constant, Ka – forward reaction rate constant described by Arrhenius equation; Pa – partial pressure of BrF3; Peq – equilibrium pressure of BrF3 above BrF3?3NaF; m – factor characterizing the interaction area of BrF3 with NaF. The application of this equation makes possible to determine the true reaction rate values and reaction activation energy in chemisorption terms; it also helps to calculate the degree BrF3 sorption on NaF reaction at different temperatures and adsorbate pressures. The possibility of sorption-desorption separation of bromine trifluoride – uranium hexafluoride – iodine pentafluoride system with use of sodium fluoride was shown.
New method of beta-NaYF4: Yb3+, Er3+ synthesis by using beta-cyclodextrin
Fedorova, Anna A.,Fedulin, Andrey I.,Morozov, Igor V.
, p. 173 - 177 (2015/08/06)
Abstract The novel method of synthesis of the hexagonal modification beta-NaYF4 doped with Yb3+ (17 at.%) and Er3+ (3 at.%) using beta-cyclodextrin is proposed. Complex fluorides were prepared by decomposition of the mixture of metal fluoroacetates hydrates with and without addition of beta-cyclodextrin and were investigated by X-ray diffraction, energy dispersive X-ray analysis, scanning and transmission electron microscopy. The samples prepared by adding beta-cyclodextrin in the reaction mixture are single phase and have a hexagonal structure. At the same time, decomposition of the mixture of metal trifluoroacetates hydrates without beta-cyclodextrin is accompanied by pyrohydrolysis, leading to a contamination of the products by impurities of yttrium oxyfluorides and sodium fluoride. So a new synthetic approach proposed in this work allows to obtain pure complex fluorides with homogeneous distribution of elements and with homogeneous pore distribution.
Study of the reaction between boron trifluoride methanol complex and sodium methoxide
Wuke, Lang,Weijiang, Zhang,Jiao, Xu,Lei, Zhang
, p. 1530 - 1540 (2014/07/08)
The reaction between boron trifluoride methanol complex and sodium methoxide in methanol solution was investigated using conductivity as the reaction indicator. The reaction conditions were examined and a mechanism of this reaction was proposed. Moreover, proper reaction conditions were proposed for boric acid preparation using this reaction. 2014