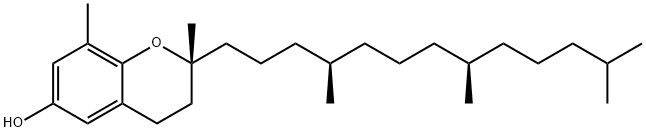
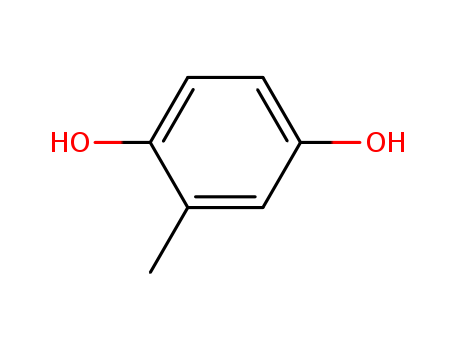
D-DELTA-TOCOPHEROL literature
CYCLIC PEROXIDE OXIDATION OF AROMATIC COMPOUND PRODUCTION AND USE THEREOF
-
Page/Page column 9; 10, (2014/10/15)
The present invention provides a method for converting an aromatic hydrocarbon to a phenol by providing an aromatic hydrocarbon comprising one or more aromatic C-H bonds and one or more activated C-H bonds in a solvent; adding a phthaloyl peroxide to the solvent; converting the phthaloyl peroxide to a di-radical; contacting the di-radical with the one or more aromatic C-H bonds; oxidizing selectively one of the one or more aromatic C-H bonds in preference to the one or more activated C-H bonds; adding a hydroxyl group to the one of the one or more aromatic C-H bonds to form one or more phenols; and purifying the one or more phenols.
Metal-free oxidation of aromatic carbon-hydrogen bonds through a reverse-rebound mechanism
Yuan, Changxia,Liang, Yong,Hernandez, Taylor,Berriochoa, Adrian,Houk, Kendall N.,Siegel, Dionicio
, p. 192 - 196 (2013/08/23)
Methods for carbon-hydrogen (C-H) bond oxidation have a fundamental role in synthetic organic chemistry, providing functionality that is required in the final target molecule or facilitating subsequent chemical transformations. Several approaches to oxidizing aliphatic C-H bonds have been described, drastically simplifying the synthesis of complex molecules. However, the selective oxidation of aromatic C-H bonds under mild conditions, especially in the context of substituted arenes with diverse functional groups, remains a challenge. The direct hydroxylation of arenes was initially achieved through the use of strong Bronsted or Lewis acids to mediate electrophilic aromatic substitution reactions with super-stoichiometric equivalents of oxidants, significantly limiting the scope of the reaction. Because the products of these reactions are more reactive than the starting materials, over-oxidation is frequently a competitive process. Transition-metal-catalysed C-H oxidation of arenes with or without directing groups has been developed, improving on the acid-mediated process; however, precious metals are required. Here we demonstrate that phthaloyl peroxide functions as a selective oxidant for the transformation of arenes to phenols under mild conditions. Although the reaction proceeds through a radical mechanism, aromatic C-H bonds are selectively oxidized in preference to activated-H bonds. Notably, a wide array of functional groups are compatible with this reaction, and this method is therefore well suited for late-stage transformations of advanced synthetic intermediates. Quantum mechanical calculations indicate that this transformation proceeds through a novel addition-abstraction mechanism, a kind of 'reverse-rebound' mechanism as distinct from the common oxygen-rebound mechanism observed for metal-oxo oxidants. These calculations also identify the origins of the experimentally observed aryl selectivity.
Process for separating tocopherol homologues
-
Page/Page column 2-3, (2008/06/13)
Methods for separating gamma and delta homologues of tocopherol are described.
The substrate specificity of tocopherol cyclase
Stocker, Achim,Fretz, Heinz,Frick, Haroun,Ruettimann, August,Woggon, Wolf-Dietrich
, p. 1129 - 1134 (2007/10/03)
The substrate specificity of the enzyme tocopherol cyclase from the blue-green algae Anabaena variabilis (Cyanobacteria) was investigated with 11 substrate analogues revealing the significance of three major recognition sites: (i) the OH group at C(1) of the hydroquinone, (ii) the (E) configuration of the double bond, and (iii) the length of the lipophilic side chain. Experiments with two affinity matrices suggest that substrates approach the enzyme's active site with the hydrophobic tail.