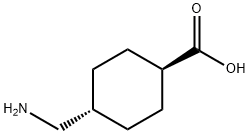
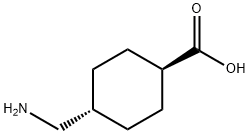
Tranexamic Acid literature
Method for synthesizing formic acid
-
, (2022/04/03)
The invention discloses a synthesis process of tranexamic acid (I), which comprises the following steps of: performing esterification reaction on 3-cyclohexene formic acid (V) serving as a raw material to obtain an intermediate 3-cyclohexene formate (IV); carrying out carbonyl insertion reaction on the intermediate (IV) and carbon monoxide/hydrogen mixed gas or synthesis gas in the presence of a catalyst to obtain an intermediate 4-formyl cyclohexane-1-formate (III) with high selectivity; carrying out reductive amination on the intermediate (III) to obtain an intermediate 4-aminomethyl cyclohexane formate (II); and finally hydrolyzing and transforming the intermediate (II) to obtain tranexamic acid (I). The raw materials are cheap and easy to obtain, the carbon monoxide carbonyl insertion reaction is green and environment-friendly, the route is simple and efficient, and a new method is provided for synthesis of tranexamic acid (I).
READY-TO-USE TRANEXAMIC ACID INTRAVENOUS SOLUTION
-
, (2020/04/09)
Ready-to-use, stable aqueous intravenous tranexamic acid compositions are provided.
A 4-acetamidomethyl methyl cyclohexyl method for producing methyl formate
-
Paragraph 0051-0053, (2017/02/28)
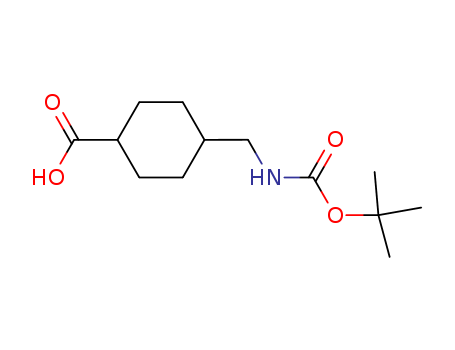
The invention relates to a preparation method of methyl 4-acetylaminomethyl hexahydrobenzoate. The method comprises the following steps: adding methyl paracyanobenzoate into a reaction kettle, adding a metal catalyst I and a cocatalyst II, heating while stirring to react under hydrogen gas conditions for 6-10 hours by using acetic anhydride as a solvent, filtering, and distilling to obtain the methyl acetylaminomethyl benzoate; and adding the obtained methyl acetylaminomethyl benzoate into a reaction kettle, adding a solvent V, a metal catalyst III and a cocatalyst IV, heating while stirring to completely react under hydrogen gas conditions, filtering, and distilling to recover the solvent, thereby obtaining the methyl 4-acetylaminomethyl hexahydrobenzoate. The synthesis technique of preparing the methyl 4-acetylaminomethyl hexahydrobenzoate from the initial raw material methyl paracyanobenzoate has the advantages of accessible raw materials, low cost, high yield and the like, and is simple to operate and suitable for industrial production. The product can be further used for preparing the trans-4-aminomethylcyclohexyl formic acid, and has huge application potential.
Design, synthesis and in vitro kinetic study of tranexamic acid prodrugs for the treatment of bleeding conditions
Karaman, Rafik,Ghareeb, Hiba,Dajani, Khuloud Kamal,Scrano, Laura,Hallak, Hussein,Abu-Lafi, Saleh,Mecca, Gennaro,Bufo, Sabino A.
, p. 615 - 635 (2013/09/02)
Based on density functional theory (DFT) calculations for the acid-catalyzed hydrolysis of several maleamic acid amide derivatives four tranexamic acid prodrugs were designed. The DFT results on the acid catalyzed hydrolysis revealed that the reaction rate-limiting step is determined on the nature of the amine leaving group. When the amine leaving group was a primary amine or tranexamic acid moiety, the tetrahedral intermediate collapse was the rate-limiting step, whereas in the cases by which the amine leaving group was aciclovir or cefuroxime the rate-limiting step was the tetrahedral intermediate formation. The linear correlation between the calculated DFT and experimental rates for N-methylmaleamic acids 1-7 provided a credible basis for designing tranexamic acid prodrugs that have the potential to release the parent drug in a sustained release fashion. For example, based on the calculated B3LYP/6-31G(d,p) rates the predicted t1/2 (a time needed for 50 % of the prodrug to be converted into drug) values for tranexamic acid prodrugs ProD 1-ProD 4 at pH 2 were 556 h [50.5 h as calculated by B3LYP/311+G(d,p)] and 6.2 h as calculated by GGA: MPW1K), 253 h, 70 s and 1.7 h, respectively. Kinetic study on the interconversion of the newly synthesized tranexamic acid prodrug ProD 1 revealed that the t1/2 for its conversion to the parent drug was largely affected by the pH of the medium. The experimental t1/2 values in 1 N HCl, buffer pH 2 and buffer pH 5 were 54 min, 23.9 and 270 h, respectively. Graphical Abstract: [Figure not available: see fulltext.]