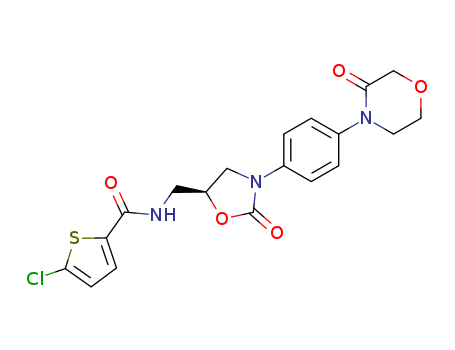
Rivaroxaban literature
New synthetic strategy for preparation of the anticoagulant drug Rivaroxaban via an asymmetric Henry reaction
Drabina, Pavel,Feixová, Viola,Sedlák, Milo?
, p. 99 - 101 (2019)
A new synthetic approach towards the anticoagulant drug (S)-Rivaroxaban was described. This reaction sequence involved six steps overall, starting from commercially available and inexpensive N-(4-aminophenyl)morpholin-3-one. The stereogenic centre was introduced by an asymmetric Henry reaction catalysed by the complex of copper(II) acetate and (2R,5S)-2-(pyridine-2-yl)imidazolidine-4-one with 87% ee. The individual reaction steps proceeded with high yields and did not require any unusual or expensive reagents.
Preparation method of rivaroxaban
-
, (2021/03/18)
The invention relates to a preparation method of rivaroxaban, belongs to the field of medicines, particularly relates to a preparation method for efficiently synthesizing rivaroxaban by taking 5-chlorothiophene-2-amide as a raw material through four steps of Boc protection, substitution reaction, cyclization reaction and deprotection reaction. The preparation method has few steps, less three wastes and good product purity.
Preparation method of rivaroxaban
-
Paragraph 0007; 0023; 0029; 0029; 0035; 0035; 0040; 0041, (2021/01/25)
The invention relates to a preparation method of rivaroxaban, in particular to a method for efficiently synthesizing rivaroxaban by taking p-nitroaniline and (S)-N-glycidol phthalimide as raw materials through seven steps: an addition reaction, a cyclization reaction, a hydrolysis reaction, an amidation reaction, a reduction reaction, an addition reaction and a cyclization reaction. The provided rivaroxaban preparation method has the advantages of high yield, low cost, few three wastes, and high product purity and is suitable for industrialization.
Preparation method of rivaroxaban
-
Paragraph 0013; 0017, (2021/03/24)
The invention provides a preparation method of a drug as shown in formula I, namely a preparation method of (S)-5-chloro-N-(((5S)-2-oxo-3-(4-(3-oxo-morpholine-4-yl) phenyl)-1, 3-oxazoline-5-yl) methyl)-thiophene-2-formamide. According to the method, the reaction steps are few, the solvent reagent consumption in the reaction process and the product loss caused by excessive operation are reduced, the yield is high, the product quality is stable, and industrial production is facilitated; and a solvent reagent is selected as a solvent reagent, so that the method is pollution-free to the environment, safe and environment-friendly, and a new method is provided for the preparation of rivaroxaban.
Synthesis method of rivaroxaban
-
Paragraph 0012; 0034-0049, (2020/07/02)
The invention discloses a synthetic method of rivaroxaban. According to the method, rivaroxaban is prepared by adopting a one-pot method; 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-1H-isoindol-1,3(2H)-one in an alcohol solvent is subjected to an ammonolysis reaction in the presence of an alkali to remove a protective group, then to evaporation to remove the alcohol solvent, and finally to condensation with 5-chlorothiophene-2-formyl chloride under the action of an acid-binding agent, wherein the reaction alcohol solvent is alcohol with a carbon number of 4 or below, the alkali is an organic alkali or inorganic alkali, a condensation reaction solvent is water, and the acid-binding agent is potassium carbonate or sodium carbonate. The method has the advantages ofmild reaction conditions, simple post-treatment, conservation of a large amount of manpower and material resources, and applicability to large-scale industrial production.