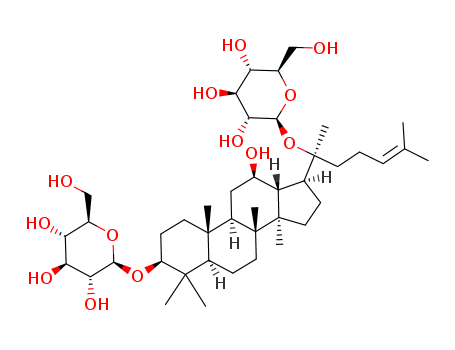
GINSENOSIDE F2 literature
Overexpression and characterization of a glycoside hydrolase family 1 enzyme from Cellulosimicrobium cellulans sp. 21 and its application for minor ginsenosides production
Yuan, Ye,Hu, Yanbo,Hu, Chenxing,Leng, Jiayi,Chen, Honglei,Zhao, Xuesong,Gao, Juan,Zhou, Yifa
, p. 60 - 67 (2015)
Abstract A novel β-glucosidase gene (ccbgl1a) was cloned from the ginsenosides-transforming strain Cellulosimicrobium cellulans sp. 21. This enzyme was overexpressed in Escherichia coli, the recombinant β-glucosidase (CcBgl1A) containing N-terminal His-tag was sufficiently purified by nickel metal affinity chromatography with purification factor of 1.9-fold and specific activity of 31.5 U/mg. The molecular mass of recombinant CcBgl1A was estimated to be approximately 46 kDa. CcBgl1A exhibited optimal activity at 35°C and pH 5.5. However, above 40°C, the enzyme stability significantly decreased. The enzyme showed high bioconversion ability on protopanaxadiol-type ginsenosides mixture (PPDGM), which could hydrolyze the outer C-3 glucose moieties of ginsenosides Rb1, Rb2, Rc and Rd into the rare ginsenosides Gypenoside XVII (Gyp XVII), compound O, ginsenoside Mb and ginsenoside F2. Scaled-up production using 1 g of the PPDGM resulted in 292 mg Gyp XVII, 134 mg CO, 184 mg Mb, and 62 mg F2, with chromatographic purities. These results suggest that CcBgl1A would be potentially useful in the preparation of pharmacologically active minor ginsenosides Gyp XVII, CO, Mb and F2.
Hydrolysis of the outer β-(1,2)-d-glucose linkage at the C-3 position of ginsenosides by a commercial β-galactosidase and its use in the production of minor ginsenosides
Kim, Yeong-Su,Kim, Do-Yeon,Kang, Dong Wook,Park, Chang-Su
supporting information, (2018/07/30)
Commercial β-galactosidase from Aspergillus oryzae (SUMILACT LTM) was used for the bioconversion of the ginsenosides Rb1, Rb2, Rc, Rd, and Rg3 to gypenoside-XVII, compound-O, compound-MC1, F2, and Rh2, respectively. The optimal conditions were
Rational design of a β-glycosidase with high regiospecificity for triterpenoid tailoring
Park, Sang Jin,Choi, Jung Min,Kyeong, Hyun-Ho,Kim, Song-Gun,Kim, Hak-Sung
, p. 854 - 860 (2015/03/30)
Triterpenoids with desired glycosylation patterns have attracted considerable attention as potential therapeutics for inflammatory diseases and various types of cancer. Sugar-hydrolyzing enzymes with high substrate specificity would be far more efficient than other methods for the synthesis of such specialty triterpenoids, but they are yet to be developed. Here we present a strategy to rationally design a β-glycosidase with high regiospecificity for triterpenoids. A β-glycosidase with broad substrate specificity was isolated, and its crystal structure was determined at 2.0 ? resolution. Based on the product profiles and substrate docking simulations, we modeled the substrate binding modes of the enzyme. From the model, the substrate binding cleft of the enzyme was redesigned in a manner that preferentially hydrolyzes glycans at specific glycosylation sites of triterpenoids. The designed mutants were shown to produce a variety of specialty triterpenoids with high purity.
Highly efficient biotransformation of ginsenoside Rb1 and Rg3 using β-galactosidase from Aspergillus sp.
Wan, Hui-Da,Li, Dan
, p. 78874 - 78879 (2015/10/05)
A preliminary study on the enzymatic biotransformation of ginsenosides is evaluated. β-Galactosidase from Aspergillus sp. displayed β-glucosidase activity, which was responsible for its ability to transform major ginsenoside Rb1 to rare ginsenoside F2 via ginsenoside Rd. The Rb1 conversion, Rd and F2 yields reached 100%, 80.7% and 14.3% after 60 h at 60 °C, respectively. Ginsenoside Rg3 can be selectively hydrolyzed and only Rh2 was obtained with this β-galactosidase as well. Before hydrolysis, an Rg3 inclusion complex was prepared with hydroxypropyl-β-cyclodextrin (HP-β-CD) to improve the aqueous solubility. The solubility of Rg3 increased 74.6 fold, and the phase solubility curve displayed a typical AL-type, which indicates the formation of a 1 : 1 inclusion complex. Using an enzyme loading of 500 U g-1 Rg3, the highest Rg3 conversion of 90.6% and Rh2 yield of 88.5% were obtained after 24 h at 60 °C. These results indicate that β-galactosidase from Aspergillus sp. could be useful for the mass production of rare ginsenosides.