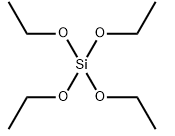
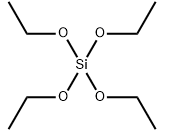
Tetraethyl orthosilicate literature
-
Ogier
, p. 118 (1879)
-
Catalysis of triethoxysilane disproportionation with oligoethylene glycol ethers
Parshina, Lidiya N.,Oparina, Lyudmila A.,Khil'ko, Marina Ya.,Trofimov, Boris A.
, p. 246 - 249 (2003)
Oligoethylene glycol ethers catalyze the disproportionation of triethoxysilane to tetraethoxysilane and silane both at room temperature and upon heating. For comparison, the CsF-catalyzed disproportionation of triethoxysilane has been examined and found m
Mechanochemical method of producing triethoxysilane
Temnikov,Anisimov,Chistovalov,Zhemchugov,Kholodkov,Zimovets,Vysochinskaya, Yu. S.,Muzafarova
, p. 270 - 274 (2019)
A mechanochemical method for synthesis of triethoxysilane from silicon-copper contact mass and ethyl alcohol in the developed vibration reactor is presented. It is shown that the process of a direct alkoxysilane synthesis in the vibro-boiling layer is affected by a series of control parameters such as the ratio between the contact mass and the mass of grinding bodies, the grinding body sizes and their ratios in a polydisperse mixture, power density. Optimization of these parameters allowed us to obtain HSi(OEt)3 with a selectivity of 50% at a silicon conversion of 90% without the use of promoters.
Method for removing methyldichlorosilane and silicon tetrachloride impurities in trimethyl chlorosilane
-
Paragraph 0037-0038; 0045-0046; 0050-0052; 0064; 0079; ..., (2021/08/25)
The invention relates to a method for removing methyldichlorosilane and silicon tetrachloride impurities in trimethyl chlorosilane, which comprises a hydrosilylation reaction, a partial esterification reaction and a complete esterification reaction. Firstly, a mixture of trimethylsilyl chloride containing methyldichlorosilane and silicon tetrachloride impurities is added to a reactor for hydrosilylation reaction, and the reaction product enters a separation system. The silicon tetrachloride in the mixture is partially esterified and reacted by adding the low-carbon alcohol as an esterifying agent, and the reaction product enters a separation system. Finally, the partially esterified product is further fully esterified to valuable tetraalkoxy silicon products. The high-efficiency recycling of trimethylchlorosilane is realized, and high-value utilization is also realized.
Sustainable Catalytic Synthesis of Diethyl Carbonate
Putro, Wahyu S.,Ikeda, Akira,Shigeyasu, Shinji,Hamura, Satoshi,Matsumoto, Seiji,Lee, Vladimir Ya.,Choi, Jun-Chul,Fukaya, Norihisa
, p. 842 - 846 (2020/12/07)
New sustainable approaches should be developed to overcome equilibrium limitation of dialkyl carbonate synthesis from CO2 and alcohols. Using tetraethyl orthosilicate (TEOS) and CO2 with Zr catalysts, we report the first example of sustainable catalytic synthesis of diethyl carbonate (DEC). The disiloxane byproduct can be reverted to TEOS. Under the same conditions, DEC can be synthesized using a wide range of alkoxysilane substrates by investigating the effects of the number of ethoxy substituent in alkoxysilane substrates, alkyl chain, and unsaturated moiety on the fundamental property of this reaction. Mechanistic insights obtained by kinetic studies, labeling experiments, and spectroscopic investigations reveal that DEC is generated via nucleophilic ethoxylation of a CO2-inserted Zr catalyst and catalyst regeneration by TEOS. The unprecedented transformation offers a new approach toward a cleaner route for DEC synthesis using recyclable alkoxysilane.
Method for preparing organosilane by utilizing organosilicone byproduct
-
Paragraph 0021-0023; 0025-0026, (2020/07/13)
The invention relates to the technical field of production of organic silicon by-products. The invention aims to solve the problems of high cost, more three wastes and continuous production of byproducts in the traditional organic silicon byproduct treatment process. The method comprises the following steps: adding the organic silicon by-product into a nitrogen-protected glass lining reaction kettle with a tower, adding a catalyst, dropwise adding alcohol to the bottom of the glass lining reaction kettle, carrying out a heating reaction under a stirring condition, neutralizing the obtained material, and rectifying the neutralized material to obtain the organic silane. According to the method, the multi-component organic silicon by-products trichlorosilane and silicon tetrachloride react and are converted into the same product, the high-purity product can be obtained only through simple rectification and purification, the process is simple, the treatment cost is low, and the product hasgood economic value.
METHOD FOR PRODUCING TETRAALKOXYSILANE
-
Paragraph 0055; 0056-0061; 0062; 0063-0066; 0069-0073, (2020/12/01)
An object of the present invention is to provide a method capable of producing a tetraalkoxysilane with a high energy efficiency and with a high yield. The present invention provides a method for producing a tetraalkoxysilane, the method including: a first step of reacting an alcohol with a silicon oxide; and a second step of bringing a vaporized component of the reaction mixture obtained in the first step into contact with a molecular sieve.