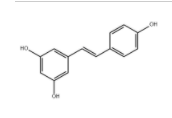
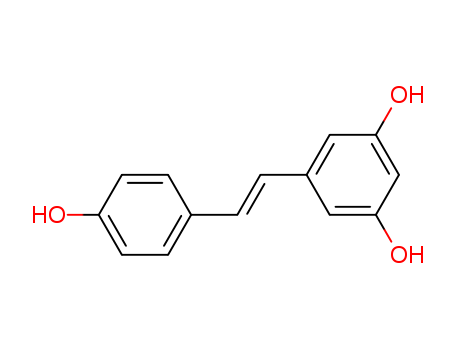
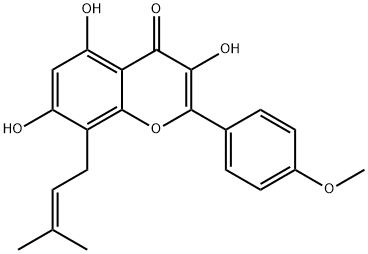
Resveratrol literature
Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cells
Zhang, Yansheng,Li, Song-Zhe,Li, Jia,Pan, Xiangqing,Cahoon, Rebecca E.,Jaworski, Jan G.,Wang, Xuemin,Jez, Joseph M.,Chen, Feng,Yu, Oliver
, p. 13030 - 13031 (2006)
Resveratrol is a naturally occurring defense compound produced by a limited number of plants in response to stresses. Besides cardiovascular benefits, this health-promoting compound has been reported to extend life spans in yeasts, flies, worms, and fish. To biosynthesize resveratrol de novo, tyrosine ammonia lyase (TAL), 4-coumarate CoA-ligase (4CL), and stilbene synthase (STS) were isolated from Rhodobacter sphaeroides, Arabidopsis thaliana, and Vitis vinifera, respectively. Yeast cells expressing 4CL and STS produce resveratrol when fed with 4-coumaric acid, the substrate of 4CL. When a translational fusion protein joining 4CL and STS was used, yeast cells produced 15-fold more resveratrol than the cotransformed cells, suggesting that physical localization of 4CL and STS facilitate resveratrol production. When the resveratrol pathway was introduced into human HEK293 cells, de novo biosynthesis was detected, leading to intracellular accumulation of resveratrol. We successfully engineered an entire plant natural product pathway into a mammalian host. Copyright
Resveratrol and arctigenin production from polydatin and arctiin respectively by a thermostable β-glucosidase from Thermotoga maritima
Xue, Yemin,Zhang, Zonghui,Hou, Jingjing,Cao, Zhigang,Zhang, Lingxian,Lou, Fen,Xu, Puxu
, p. 414 - 430 (2018)
For preparation of resveratrol and arctigenin from peanut hulls and arctium lappa fruits, respectively, a recombinant β-glucosidase (TmBglA) from hyperthermophile Thermotoga maritima was purified and characterized. The hydrolytic activity was the highest at 90 °C and pH 6.2 for arctiin with Km of 1.61 mM and kcat of 197.4 s?1, and 90 °C and 5.8 for polydatin with Km of 0.38 mM and kcat of 47.6 s?1. The enzyme produced 215.4 mg L?1 resveratrol and 355.7 mg L?1 arctigenin from 400 mg L?1 polydatin and 540 mg L?1 arctiin after 60 min of incubation at 80 °C, with capable of hydrolyzing up to 92.1 and 94.9% of polydatin and arctiin, respectively. The enzymatic hydrolysis of peanut hulls and fructus arctii displayed a conversion yield of 3.8 and 0.33 mg resveratrol and arctigenin per gram of substrate material flour, respectively. Of the reported β-glucosidase, TmBglA exhibited the highest thermostability, kcat, kcat/Km, and conversion productivity for hydrolyzing polydatin and arctiin, and has great potential applications in functional food and medicine production.
An innovative biotransformation to produce resveratrol by: Bacillus safensis
Hu, Xiaoyan,Liu, Yexue,Li, Dengke,Feng, Wei,Ni, Hanmeng,Cao, Shan,Lu, Fuping,Li, Yu
, p. 15448 - 15456 (2019)
Resveratrol is considered as a potential food supplement, cosmetic ingredient and nutraceutical. In this study, resveratrol was produced by biotransformation successfully. In detail, a β-glucosidase producing strain was isolated and identified as Bacillus safensis, and it could convert polydatin to resveratrol efficiently and rapidly. Further research showed that the conversion rate to resveratrol reached 93.1% in 8 h at 37 °C. The production of resveratrol was confirmed by HPLC, LC-MS and 1H-NMR to identify its structure and it was verified to possess antibacterial properties especially against Escherchia coli. To illustrate the resveratrol transformation mechanism, several glucosidases from B. safensis CGMCC 13129 were expressed and analyzed. The results showed that BGL4 and BGL5 had higher transformation activity compared with other tested glucosidases. This research provides a novel approach to produce resveratrol, and would promote the application of resveratrol in health-promoting pharmaceutical and food products.
The occurrence of piceid, a stilbene glucoside, in grape berries
Waterhouse,Lamuela-Raventos
, p. 571 - 573 (1994)
-
Identification of metabolic pattern and bioactive form of resveratrol in human medulloblastoma cells
Shu, Xiao-Hong,Li, Hong,Sun, Zheng,Wu, Mo-Li,Ma, Jing-Xin,Wang, Jian-Min,Wang, Qian,Sun, Yuan,Fu, Yuan-Shan,Chen, Xiao-Yan,Kong, Qing-You,Liu, Jia
, p. 1516 - 1525 (2010)
Cancer preventive reagent trans-resveratrol is intracellularly biotransformed to different metabolites. However, it is still unclear whether trans-resveratrol exerts its biological effects directly or through its metabolite(s). This issue was addressed here by identifying the metabolic pattern and the bioactive form of resveratrol in a resveratrol-sensitive human medulloblastoma cell line, UW228-3. The cell lysates and condition media of UW228-3 cells with or without 100 μM resveratrol treatment were analyzed by HPLC and LC/MS which revealed (1) that resveratrol was chemically unstable and the spontaneous generation of cis-resveratrol reduced resveratrol's anti-medulloblastoma efficacy and (2) that resveratrol monosulfate was the major metabolite of the cells. To identify the bioactive form of resveratrol, a mixture-containing approximately half fraction of resveratrol monosulfate was prepared by incubating trans-resveratrol with freshly prepared rat brain lysates. Medulloblastoma cells treated by 100 μM of this mixture showed attenuated cell crisis. The overall levels of the three brain-associated sulfotransferases (SULT1A1, 1C2 and 4A1) were low in medulloblastoma cells in vivo and in vitro in comparison with that in human noncancerous and rat normal cerebella; resveratrol could more or less up-regulate the production of these enzymes in UW228-3 cells but their overall level was still lower than that in normal cerebellum tissue. Our study thus demonstrated for the first time that trans-resveratrol is the bioactive form in medulloblastoma cells in which the expression of brain-associated SULTs was down-regulated, resulting in the increased intracellular bioavailability and anti-medulloblastoma efficacy of trans-resveratrol.
Synthesis and Evaluation as Prodrugs of Hydrophilic Carbamate Ester Analogues of Resveratrol
Azzolini, Michele,Mattarei, Andrea,La Spina, Martina,Marotta, Ester,Zoratti, Mario,Paradisi, Cristina,Biasutto, Lucia
, p. 3441 - 3454 (2015)
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is an unfulfilled promise for health care: its exploitation is hindered by rapid conjugative metabolism in enterocytes and hepatocytes; low water solubility is a serious practical problem. To advantageously modify the physicochemical properties of the compound we have developed prodrugs in which all or part of the hydroxyl groups are linked via an N-monosubstituted carbamate ester bond to promoieties derived from glycerol or galactose, conferring higher water solubility. Kinetic studies of hydrolysis in aqueous solutions and in blood indicated that regeneration of resveratrol takes place in an appropriate time frame for delivery via oral administration. Despite their hydrophilicity some of the synthesized compounds were absorbed in the gastrointestinal tract of rats. In these cases the species found in blood after administration of a bolus consisted mainly of partially deprotected resveratrol derivatives and of the products of their glucuronidation, thus providing proof-of-principle evidence of behavior as prodrugs. The soluble compounds largely reached the lower intestinal tract. Upon administration of resveratrol, the major species found in this region was dihydroresveratrol, produced by enzymes of the intestinal flora. In experiments with a fully protected (trisubstituted) deoxygalactose containing prodrug, the major species were the prodrug itself and partially deprotected derivatives, along with small amounts of dihydroresveratrol. We conclude that the N-monosubstituted carbamate moiety is suitable for use in prodrugs of polyphenols. (Chemical Equation Presented).
Tandem Acceptorless Dehydrogenative Coupling-Decyanation under Nickel Catalysis
Babu, Reshma,Balaraman, Ekambaram,Midya, Siba P.,Subaramanian, Murugan,Yadav, Vinita
, p. 7552 - 7562 (2021/06/28)
The development of new catalytic processes based on abundantly available starting materials by cheap metals is always a fascinating task and marks an important transition in the chemical industry. Herein, a nickel-catalyzed acceptorless dehydrogenative coupling of alcohols with nitriles followed by decyanation of nitriles to access diversely substituted olefins is reported. This unprecedented C=C bond-forming methodology takes place in a tandem manner with the formation of formamide as a sole byproduct. The significant advantages of this strategy are the low-cost nickel catalyst, good functional group compatibility (ether, thioether, halo, cyano, ester, amino, N/O/S heterocycles; 43 examples), synthetic convenience, and high reaction selectivity and efficiency.
Pd-Catalyzed Cross-Coupling of Organostibines with Styrenes to Give Unsymmetric (E)-Stilbenes and (1 E,3 E)-1,4-Diarylbuta-1,3-dienes and Fluorescence Properties of the Products
Zhang, Zhao,Zhang, Dejiang,Zhu, Longzhi,Zeng, Dishu,Kambe, Nobuaki,Qiu, Renhua
supporting information, p. 5317 - 5322 (2021/06/28)
A general and effective palladium-catalyzed cross-coupling of organostibines with styrenes to give (E)-olefins was disclosed. By the use of an organostibine reagent, this method can produce unsymmetric (E)-1,2-diarylethylenes and (1E,3E)-1,4-diarylbuta-1,3-dienes in good yields with high E/Z selectivity and good functional group tolerance. Resveratrol and DMU-212 were synthesized in high yield. The protocol can be extended to the synthesis of (1E,3E,5E)-1,6-diphenylhexa-1,3,5-triene in 40% yield. Products 5e, 5f, and 7a showed good photoluminescence quantum yields ranging from 72 to 99%.
N-Heterocyclic carbene palladium (II)-pyridine (NHC-Pd (II)-Py) complex catalyzed heck reactions
Li, Dan,Tian, Qingqiang,Wang, Xuetong,Wang, Qiang,Wang, Yin,Liao, Siwei,Xu, Ping,Huang, Xin,Yuan, Jianyong
supporting information, p. 2041 - 2052 (2021/05/25)
A mild, efficient, and practical catalytic system for the synthesis of highly privileged stilbene pharmacophores is reported. This system uses N-heterocyclic carbene palladium (II) Pyridine (NHC-Pd (II)-Py) complex to catalyze the formation of carbon-carbon bonds between olefin derivatives and various bromide. This simple, gentle and user-friendly method can offer a variety of stilbene products in excellent yields under solvent-free condition. And its scale-up reaction has excellent yield and this system can be applied to industrial fields. The utility of this method is highlighted by its universality and modular synthesis of a series of bioactive molecules or important medical intermediates.
Synthesis of Stilbenes by Rhodium-Catalyzed Aerobic Alkenylation of Arenes via C-H Activation
Jia, Xiaofan,Frye, Lucas I.,Zhu, Weihao,Gu, Shunyan,Gunnoe, T. Brent
supporting information, p. 10534 - 10543 (2020/06/08)
Arene alkenylation is commonly achieved by late transition metal-mediated C(sp2)-C(sp2) cross-coupling, but this strategy typically requires prefunctionalized substrates (e.g., with halides or pseudohalides) and/or the presence of a directing group on the arene. Transition metal-mediated arene C-H activation and alkenylation offers an alternative method to functionalize arene substrates. Herein, we report a rhodium-catalyzed oxidative arene alkenylation from arenes and styrenes to prepare stilbene and stilbene derivatives. The reaction is successful with several functional groups on both the arene and the olefin including fluoride, chloride, trifluoromethyl, ester, nitro, acetate, cyanide, and ether groups. Reactions of monosubstituted arenes are selective for alkenylation at the meta and para positions, generally with approximately 2:1 selectivity, respectively. Resveratrol and (E)-1,2,3-trimethoxy-5-(4-methoxystyryl)benzene (DMU-212) are synthesized by this single-step approach in high yield. Comparison with palladium catalysis showed that rhodium catalysis is more selective for meta-functionalization for monosubstituted arenes and that the Rh catalysis has better tolerance of halogen groups.