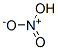Nitric acid literature
-
Bonner,Lockhart
, p. 3858,3861 (1958)
-
Formation and Decay of Peroxynitrous Acid: A Pulse Radiolysis Study
Loegager, T.,Sehested, K.
, p. 6664 - 6669 (1993)
Peroxynitrous acid and peroxynitrite anion have been studied using pulse radiolysis of nitrite and nitrate solutions.The formation rate constant determined to be k(OH + NO2) = (4.5 +/- 1.0) * 109 M-1 s-1, and the rate constant for the OH radical reaction with nitrite is determined to be k(OH +NO2-) = (6.0 +/- 1.0) * 109 M-1 s-1.In nitrate solutions, the competing reaction between OH and NO32- is found to have a rate constant of k(OH + NO32- = (3.0 +/- 1.0) * 109 M-1 s-1.The intermediate species in the nitrate system, NO32-, HNO3-, and H2NO3, decay into NO2 according to the first-order rate constants: (5.6 +/ - 0.5) * 104, (2.0 +/- 0.5) * 105, and (7.0 +/- 2.0) * 105 s-1, respectively.The rate constants k(H + NO3-) = (1.0 +/- 0.3) * 107 M-1 s-1 and k(H + NO2) = (1.0 +/- 0.2) * 1010 M-1 s-1 were also determined.The pKa of NOOH is found to be 6.5 +/- 0.1 by absorption measurements, and the maximum extinction coefficient at 240 nm is ε240(ONOOH) = 770 +/- 50 M-1 cm-1.The decay of peroxynitrous acid is detrmined to proceed through the first-order isomerization of ONOOH to HNO3 according to the rate equation kobs = kiso/(1 + Ka/+>) with rate constants kiso = 1.0 +/- 0.2 s-1 and Ka = (1.0 +/- 0.3) * 10-7.A comparison of all available literature values for the pKa and the decay rate is reported.
Visible light photocatalytic degradation of nitric oxides on PtOx-modified TiO2 via sol-gel and impregnation method
Huang, Chun-Hung,Wang, I-Kai,Lin, Yu-Ming,Tseng, Yao-Hsuan,Lu, Chun-Mei
, p. 163 - 170 (2010)
The visible light active catalysts, PtOx-doped TiO2 (PtOx-TiO2) and PtOx-loaded TiO2 (PtOx/TiO2), were successfully synthesized by the acid-catalyzed sol-gel process and the impregnation method. Pt(NH3)4(NO3)2 or H2Pt(OH)6 was employed as the PtOx precursor. By comparing the results of De-NOx, the modified photocatalysts exhibited a higher visible-light-responsive activity, and a lower NO2 selectivity than the unmodified ones. The FE-SEM images suggested that the particle size was unchanged after modification. The XRD patterns showed that the crystal structure still remained as anatase phase. Nitrogen adsorption revealed no significant change in surface areas for all samples. The UV-vis spectra indicated that PtOx promoted the absorption of visible light. Furthermore, the XPS spectra evidenced that the mixed valence states of PtO-PtO2 coexisted on the surface of TiO2. The adding of PtOx on TiO2 not only promoted the visible-light-responsive activity of converting NO to NO2 but also increased the consecutive reaction rate of NO2 to NO3-.
The inhibition of N2O5 hydrolysis in sulfuric acid by 1-butanol and 1-hexanol surfactant coatings
Park, Seong-Chan,Burden, Daniel K.,Nathanson, Gilbert M.
, p. 2921 - 2929 (2007)
Gas - liquid scattering experiments are used to measure the fraction of N2O5 molecules that are converted to HNO3 after colliding with 72 wt % H2SO4 containing 1-hexanol or 1-butanol at 216 K. These alcohols segregate to the surface of the acid, with saturation coverages estimated to be 60% of a close-packed monolayer for 1-hexanol and 44% of a close-packed monolayer for 1-butanol. We find that the alkyl films reduce the conversion of N2O5 to HNO 3 from 0.15 on bare acid to 0.06 on the hexyl-coated acid and to 0.10 on the butyl-coated acid. The entry of HC1 and HBr, however, is enhanced by the hexanol and butanol films. The hydrolysis of N2O5 may be inhibited because the alkyl chains restrict the transport of this large molecule and because the alcohol OH groups dilute the surface region, suppressing reaction between N2O5 and near-interfacial H 3O+ or H2O. In contrast, the interfacial alcohol OH groups provide additional binding sites for HC1 and HBr and help initiate ionization. These and previous scattering experiments indicate that short-chain alcohol surfactants impede or enhance sulfuric acid-mediated reactions in ways that depend on the chain length, liquid phase acidity, and nature of the gas molecule.
Photocatalytic activity of silicon-based nanoflakes for the decomposition of nitrogen monoxide
Itahara, Hiroshi,Wu, Xiaoyong,Imagawa, Haruo,Yin, Shu,Kojima, Kazunobu,Chichibu, Shigefusa F.,Sato, Tsugio
, p. 8643 - 8648 (2017)
The photocatalytic decomposition of nitrogen monoxide (NO) was achieved for the first time using Si-based nanomaterials. Nanocomposite powders composed of Si nanoflakes and metallic particles (Ni and Ni3Si) were synthesized using a simple one-pot reaction of layered CaSi2 and NiCl2. The synthesized nanocomposites have a wide optical absorption band from the visible to the ultraviolet. Under the assumption of a direct transition, the photoabsorption behavior is well described and an absorption edge of ca. 1.8 eV is indicated. Conventional Si and SiO powders with indirect absorption edges of 1.1 and 1.4 eV, respectively, exhibit considerably low photocatalytic activities for NO decomposition. In contrast, the synthesized nanocomposites exhibited photocatalytic activities under irradiation with light at wavelengths >290 nm (<4.28 eV). The photocatalytic activities of the nanocomposites were confirmed to be constant and did not degrade with the light irradiation time.
An Upper Limit to the Rate of the HCl + ClONO2 Reaction
Molina, L. T.,Molina, M. J.,Stachnik, R. A.,Tom, R. D.
, p. 3779 - 3781 (1985)
The reaction HCl + ClONO2 -> Cl2 + HNO3 has been studied by FTIR spectroscopy and by a static wallless UV absorption technique.An upper limit to the homogeneous bimolecular rate constant of 1E-19 cm3 molecule-1 s-1 was established, making the reaction unimportant in the stratosphere.
Comparison of zinc oxide nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue
Jang, Young Joon,Simer, Cynthia,Ohm, Taein
, p. 67 - 77 (2006)
Comparison of ZnO nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue was investigated. ZnO nanoparticles and its nano-crystalline particles were synthesized from sprayed droplets of an aqueous zinc nitrate solution by flame spray pyrolysis and spray pyrolysis assisted with an electrical furnace, respectively. ZnO nanoparticles of 20 nm in average diameter and ZnO nano-crystalline particles of 20 nm in the grain size were prepared to compare the photocatalytic activity. The photocatalytic activity of those ZnO particles was evaluated by measuring the degradation of methylene blue in water under the illumination of ultraviolet rays. Effect of the particle morphology, initial concentration of methylene blue, and photocatalyst loading on the degradation of the methylene blue was investigated under the illumination of ultraviolet rays. The photocatalytic degradation capacity of the ZnO nanoparticles was higher than that of the ZnO nano-crystalline particles. The efficiency of photocatalytic degradation of methylene blue increased with increase in photocatalyst loading and decrease in initial concentration regardless of particle morphology.
Water vapor effect on the HNO3 yield in the HO2 + NO reaction: Experimental and theoretical evidence
Butkovskaya, Nadezhda,Rayez, Marie-Therese,Rayez, Jean-Claude,Kukui, Alexandre,Le Bras, Georges
, p. 11327 - 11342 (2009)
The influence of water vapor on the production of nitric acid in the gas-phase HO2 + NO reaction was determined at 298 K and 200 Torr using a high-pressure turbulent flow reactor coupled with a chemical ionization mass spectrometer. The yield o
Molecular Complexes of Nitric Acid with N2, CO and NO studied by Matrix Isolation IR Spectroscopy
Barnes, Austin J.,Lasson, Emilie,Nielsen, Claus J.
, p. 3111 - 3116 (1995)
The interaction of nitric acid with dinitrogen, carbon monoxide and nitric oxide has been investigated by IR spectroscopy in low-temperature argon matrices.The spectra show that under these conditions N2 interacts strongly and specifically with HNO3, forming several distinct 1 : 1 complexes.The probable structures of these complexes are discussed.CO behaves in a similar manner to N2, forming complexes of the type <*>-ONO2; weak bands due to a <*>-ONO2 complex were generated by photolysis.For NO, complexes of HNO3 were observed with both monomeric and dimeric NO.The strength of interaction with HNO3 was found to increase in the order N2 < NO < CO.
Ferric microperoxidase-11 catalyzes peroxynitrite isomerization
Ascenzi, Paolo,Leboffe, Loris,Santucci, Roberto,Coletta, Massimo
, p. 56 - 61 (2015)
Microperoxidase-11 (MP11) is an undecapeptide derived from horse heart cytochrome c offering the possibility to study the reactivity of the heme group relatively unshielded by the protein. Here, the peroxynitrite isomerization to NO3-
Exploration of the Mechanism of the Activation of ClONO2 by HCl in Small Water Clusters Using Electronic Structure Methods
McNamara, Jonathan P.,Tresadern, Gary,Hillier, Ian H.
, p. 4030 - 4044 (2000)
High-level electronic structure calculations were used to study the mechanism of the reaction of ClONO2 with HCl in neutral water clusters containing one to five solvating water molecules. For the reaction between molecular HCl and ClONO2, the barrier decreases from 42 kcal mol-1 (uncatalyzed) to essentially zero when catalyzed by only two water molecules, where the reaction products involve Cl2 and HONO2. The calculations thus predict that the gas-phase reaction may be important in the stratospheric reactivation of ClONO2. The reaction between ClONO2 and solvated H3O+Cl-, as on the polar stratospheric cloud (PSC) surface, was investigated with clusters involving up to seven water molecules. The ice-catalyzed reaction involves an ionic mechanism whereby charge transfer to ClONO2 from the attacking nucleophile leads to significant ionization along the Cl-ONO2 bond. The effect of the size of the first solvation shell of Cl- is addressed by our calculations. In a cluster containing three waters and a five-water cluster structurally related to hexagonal ice, ClONO2 reacts spontaneously with HCl to yield Cl2/HONO2 in the three-water reaction and Cl2/H3O+-NO3- in the five-water-catalyzed reaction. The calculations thus predict that the reaction of ClONO2 with HCl on PSC ice aerosols can proceed spontaneously via an ionic pathway.
The hydrolysis of chlorine nitrate and its possible atmospheric significance
Rowland,Sato,Khwaja,Elliott
, p. 1985 - 1988 (1986)
The hydrolysis of CIONO2 takes place very readily on a variety of laboratory surfaces and may also occur catalytically on particulate surfaces in the stratosphere. The reaction can be considered as an oxide exchange between two X-O-Y molecules with X and Y = H, C1, or NO2. Two other reactions in this class which might occur in the stratosphere are HOCl plus HOCl, and HOCl plus ClONO2. Each of these three is approximately thermoneutral and should be accompanied by the reverse reaction with a comparable reaction rate constant. Current atmospheric models have not explained the very large ozone depletions which have taken place during Antarctic spring in the past decade. The chemical reactions included in these models may need to include heterogeneous catalysis of one or more of these oxide exchange reactions.
Kinetic Study of the Autocatalytic Nitric Acid-Bromide Reaction and Its Reverse, the Nitrous Acid-Bromine Reaction
Lengyel, Istvan,Nagy, Istvan,Bazsa, Gyoergy
, p. 2801 - 2807 (1989)
The kinetics of the forward and backward processes of the reaction NO3- + 2 Br- + 3 H+ <-> Br2 + HNO2 + H2O have been studied.The overall process is true dynamic equilibrium.The same equilibrium position can be reached fro
The Heterogeneous Reaction of N2O5 with HBr on Ice Comparison with N2O5+HCl
Seisel, Sabine,Miche, Beno?t Flilckiger,Rossi
, p. 811 - 820 (1998)
The heterogeneous reactions of N2O5 with HC1 and HBr on ice have been studied in the temperature range 180 to 200 K using a Knudsen flow reactor. The uptake of N2O5 on ice in the presence of HBr was found to be strongly dependent on :he HBr concentration. For the highest HBr concentrations used a maximum uptake coefficient of N2O5 of 7 = 0.11 has been determined. We observed Br2 and MONO in 80% yield as products with respect to N2O5 taken up. The uptake coefficient of N2O5 on ice in the presence of HC1 was found to be 3.2-10-2 and increased with increasing HCl concentration. C1NO2 was detected as the sole reaction product with a maximum yield of 63% with respect to N2O5 consumed. Hydrolysis of N2O5 resulting in HNO3 was found to be competitive with the title reaction. For the case of the HBr reaction the branching ratio between Br2 and HONO formation, on the one hand, and hydrolysis of N2O5, on the other hand, has been determined. Mechanistic aspects of the heterogeneous reaction of N2O5 with HX have been discussed. WILEY-VCH Verlag GmbH, 1998.
Reactivity of ClONO2 on H2(18)O Ice and Organic Liquids
Hanson, D. R.
, p. 13059 - 13061 (1995)
The reactive uptake of ClONO2 onto 18O-labeled ice and onto organic liquids was measured in a cylindrical flow tube reactor using chemical ionization detection.The hydrolysis of ClONO2 on H2(18)O ice produced primarily H(18)OCl, indicating that the Cl-ONO2 bond is broken upon hydrolysis on ice.The loss of ClONO2 onto liquid organic surfaces (ethylene glycol, cyclohexanone, decanol, and tridecane) was found to be efficient (reaction probability > 0.06), and the product HNO3 was detected in the gas phase.This suggests that dissociation or ionization are not prerequisites for heterogeneous reactions of ClONO2.
Evaluation of Activation Volumes for the Conversion of Peroxynitrous to Nitric Acid
Kissner, Reinhard,Thomas, Chris,Hamsa, Mohamed S.A.,Van Eldik, Rudi,Koppenol, Willem H.
, p. 11261 - 11263 (2003)
Peroxynitrous acid, an inorganic toxin of biological importance, acts both as an oxidizing and a nitrating agent during its conversion to nitric acid. In discussions of the mechanism of this conversion, activation volumes have been invoked to distinguish between possible mechanisms, viz., homolysis of the O-O bond versus rotation via the N-O bond of peroxynitrous acid. A reinvestigation of the activation volume for the conversion of peroxynitrous acid to nitric acid by high-pressure stopped-flow spectrophotometry yielded an average value of 6.9 ± 0.9 cm3 mol-1 at 25 °C. Activation volumes currently cited in the literature for this process range from 6 to 10 cm3 mol-1 in the temperature range 18-25 °C. Such moderately positive values do not support a definite conclusion regarding the mechanism of the conversion.
ZIF-67/CoOOH cocatalyst modified g-C3N4 for promoting photocatalytic deep oxidation of NO
Du, Guangzhi,Huang, Zeai,Li, Bangxin,Wang, Dajun,Xiao, Wenyan,Yi, Zeyu,Zhang, Qian,Zhao, Hongtao,Zheng, Qian,Zhu, Lin,Zou, Yanzhao
, (2021)
The removal of nitrogen oxides (NOX) by semiconductor photocatalysis is an emerging technology in recent years. However, due to incomplete oxidation, the photocatalytic oxidation of NOX is usually accompanied by the generation of toxic intermediate by-products nitrogen dioxide (NO2), which causes secondary pollution and seriously limits its practical application. To tackle the issue, ZIF-67/CoOOH (ZIF-CH) cocatalyst was constructed via flexible strongly alkali oxidation treatment to modify g-C3N4, in which ZIF-67 was selected as a cobalt source of CoOOH. XRD, SEM, TEM and XPS demonstrated the ZIF-CH was successfully synthesized and anchored on CN. UV–vis, PL, EIS, transfer photocurrent response and DFT indicated that the introduction of ZIF-CH enlarged the response range to visible light, favored the separation and transfer of carriers and improved NO/NO2 adsorption ability. Consequently, the optimized ZIF-67/CoOOH/g-C3N4 (ZIF-CH/CN) exhibited a superior NO removal efficiency of 52.5% without any generation of toxic by-product NO2, and the cycling tests indicated the high stability of ZIF-CH/CN was obtained. In-suit DRIFTS and ESR were used to investigate the reaction pathway by comparing adsorption energy and detecting the reaction intermediates and products. More importantly, this result reveal that amount of hydroxyl radical (·OH) increased after introducing ZIF-CH cocatalyst, which promotes the deep oxidation of NO. These findings could supply a convenient and effective strategy for the design of a cocatalyst to enhance the photocatalytic oxidation performance of NO and inhibit the production of toxic by-product NO2.
Heterogeneous Reactions on Model Polar Stratospheric Cloud Surfaces: Reaction of N2O5 on Ice and Nitric Acid Trihydrate
Quinlan, Michael A.,Reihs, Christa M.,Golden, David M.,Tolbert, Margaret A.
, p. 3255 - 3260 (1990)
A Knudsen cell flow reactor was used to study the heterogeneous reaction of N2O5 on laboratory ice surfaces and nitric acid trihydrate (NAT) surfaces representative of polar stratospheric clouds (PSCs).N2O5 was quantitatively converted to HNO3 on ice surfaces at 188 K.On initially pure ice surfaces, a gradual increase in the N2O5 uptake efficiency was observed up to a maximum value near 0.03.The slow rise in reactivity with time is consistent with an acid-catalyzed surface reaction.With increasing initial nitric acid concentrations, the maximum reactivity occurred more rapidly although the overall reactivity was depressed.The uptake efficiency for N2O5 on NAT at 188 K was found to be 0.015 +/- 0.006.
Infrared matrix isolation and theoretical studies of SO2-HNO3 and SO2-HONO systems
Wierzejewska, Maria,Mielke, Zofia,Wieczorek, Robert,Latajka, Zdzislaw
, p. 17 - 29 (1998)
Argon matrix infrared spectra of sulphur dioxide complexes with nitric or nitrous acid indicate formation of hydrogen-bonded structures. The red shifts of the OH stretching modes are equal to ca. 179, 51 and 40 cm-1 in SO2-HNO3<
Kinetics of N2O5 hydrolysis on secondary organic aerosol and mixed ammonium bisulfate-secondary organic aerosol particles
Escorcia, Egda N.,Sjostedt, Steven J.,Abbatt, Jonathan P.D.
, p. 13113 - 13121 (2010)
The kinetics of the hydrolysis reaction of N2O5 on secondary organic aerosol (SOA) produced through the ozonolysis of α-pinene and on mixed ammonium bisulfate-SOA particles was investigated using an entrained aerosol flow tube coupled to a chemical ionization mass spectrometer. We report room temperature uptake coefficients, γ, on ammonium bisulfate and SOA particles at 50% relative humidity of 1.5 × 10-2 ± 1.5 × 10-3 and 1.5 × 10 -4 ± 2 × 10-5, respectively. For the mixed ammonium bisulfate-SOA particles, γ decreased from 2.6 × 10 -3 ± 4 × 10-4 to 3.0 × 10-4 ± 3 × 10-5 as the SOA mass fraction increased from 9 to 79, indicating a strong suppression in γ with the addition of organic material. There is an order-of-magnitude reduction in the uptake coefficient with the smallest amount of SOA material present and smaller additional reductions with increasing aerosol organic content. This newly coated organic layer may either decrease the mass accommodation coefficient of N 2O5 onto the particle or hinder the dissolution and diffusion of N2O5 into the remainder of the aerosol after it has been accommodated onto the surface. The former corresponds to a surface effect and the latter to bulk processes. The low value of the uptake coefficient on pure SOA particles will likely make N2O5 hydrolysis insignificant on such an aerosol, but atmospheric chemistry models need to account for the role that organics may play in suppressing the kinetics of this reaction on mixed organic-inorganic particles.
UV Resonance Raman Investigation of Pentaerythritol Tetranitrate Solution Photochemistry and Photoproduct Hydrolysis
Gares, Katie L.,Bykov, Sergei V.,Asher, Sanford A.
, p. 7889 - 7894 (2017)
Ultraviolet resonance Raman spectroscopy (UVRR) is being developed for standoff trace explosives detection. To accomplish this, it is important to develop a deep understanding of the accompanying UV excited photochemistry of explosives, as well as the impact of reactions on the resulting photoproducts. In the work here we used 229 nm excited UVRR spectroscopy to monitor the photochemistry of pentaerythritol tetranitrate (PETN) in acetonitrile. We find that solutions of PETN in CD3CN photodegrade with a quantum yield of 0.08 ± 0.02, as measured by high performance liquid chromatography (HPLC). The initial step in the 229 nm UV photolysis of PETN in CD3CN is cleavage of an O-NO2 bond to form NO2. The accompanying photoproduct is pentaerythritol trinitrate (PETriN), (CH2ONO2)3CCH2OH formed by photolysis of a single O-NO2. The resulting UVRR spectra show a dominant photoproduct band at ~1308 cm-1, which derives from the symmetric stretch of dissolved NO2. This photoproduct NO2 is hydrolyzed by trace amounts of water, which downshifts this 1308 cm-1 NO2 Raman band due to the formation of molecular HNO3. The dissociation of HNO3 to NO3- in the presence of additional water results in an intense NO3- symmetric stretching UVRR band at 1044 cm-1.
Studies on the characterization of novel Eu(III) complexes with β-diketones and diaza-18-crown-6
Yu, Jiang,Xu, Zhenhua,Guo, Jingyong,Xu, Guangxian
, p. 1183 - 1198 (1999)
-
The oxidation product (NO3-) of NO pollutant in flue gas used as a nitrogen source to improve microalgal biomass production and CO2fixation
Cheng, Jun,Huang, Yun,Lu, Hongxiang,Huang, Rui,Zhou, Junhu,Cen, Kefa
, p. 42147 - 42154 (2014)
In order to eliminate the inhibition effect of the toxic nitric oxide (NO) in flue gas on microalgal growth and CO2fixation, NO was converted by a wet UV/H2O2method to produce nitrate (NO3-), which then be used as a nitrogen source for microalgae to improve its growth. The growth ability and biomass compositions of the microalgae cultivated with the produced NO3-from NO gas were similar to those of the microalgae cultivated with equivalent moles of commercial NaNO3. The NO3-concentration produced from NO increased with UV lamp power, H2O2, and NO concentrations, resulting in an improved microalgal growth. The concentration of NO3-from 500 ppm NO wet-oxidized by 6% (v/v) H2O2and 55 W UV light was up to 8.8 mM. When the produced nitrate was used as supplementary nitrogen source, the maximum growth productivity of Chlorella PY-ZU1 at 15% (v/v) CO2reached 1.18 g L-1per day (0.97 times higher than that cultivated with the standard medium). The peak fixation efficiency of 15% (v/v) CO2was 69.6% (1.13 times higher than that cultivated with the standard medium). This journal is
Nitric acid uptake and decomposition on black carbon (soot) surfaces: Its implications for the upper troposphere and lower stratosphere
Choi, Wonyong,Leu, Ming-Taun
, p. 7618 - 7630 (1998)
The uptake and decomposition of HNO3 on black carbon (soot) surfaces were investigated in order to evaluate the proposal that HNO3 decomposition on aircraft-generated soot aerosols may alter the NOx/NOy partitioning in the upper troposphere and lower stratosphere. The experimental measurements were performed by using a fast flow-tube reactor coupled to a quadrupole mass spectrometer. Black carbon samples used as surrogate material for aircraft soot in this study included Degussa FW2 (an amorphous carbon black comprising medium oxides), graphite, hexane soot, and kerosene soot. The measurements of uptake were performed by varying P(HNO3) in the range of 5 × 10-7 to 5 × 10-4 Torr at 220 and 295 K. The results are summarized as follows. Significant HNO3 decomposition was observed on FW2 at 295 K with P(HNO3) ≥ 1 × 10-4 Torr, while it did not occur at 220 K. Similar HNO3 decomposition behavior on graphite was also observed under the condition of P(HNO3) ≥ 10-4 Torr and T = 295 K, although the extent of the decomposition was much smaller than that on FW2. The decomposition of HNO3 on soot produced NO, NO2, H2O, oxidized soot surface, and some unidentified volatile products. To explain the observed decomposition behavior at higher partial pressures of HNO3, a bimolecular HNO3 decomposition mechanism on soot surfaces was proposed. However, HNO3 immediately decomposed on an FW2 surface at 503 K even at lower partial pressure (~10-6 Torr). On flame-deposited hexane and kerosene soot film, no HNO3 decomposition was observed up to P(HNO3) = 5 × 10-4 Torr. Moreover, the uptake and desorption of HNO3 were reversible at 295 K and irreversible at 220 K. Adsorbed HNO3 molecules on hexane soot film were saturated to a monolayer coverage at P(HNO3) ~ 2 × 10-4 Torr according to Langmuir adsorption isotherm; further increase in P(HNO3) resulted in multilayer adsorption. Under the experimental conditions (P(HNO3) = 5 × 10-7 Torr and T = 220 K), the uptake of HNO3 was found to involve purely physical adsorption without showing any sign of irreversible decomposition over all black carbon samples. Subsequent heating of the sample following the uptake at 220 K desorbed most of the adsorbed HNO3 molecules. Physical adsorption of HNO3 was found to take place on the surface of concentrated H2SO4-coated soot at 230 K, but decomposition of HNO3 took place at 296 K. Finally, the present results suggest that the HNO3 decomposition on soot aerosols through a direct gas-solid interaction, which was proposed as a possible NOy-reactivation mechanism in the atmospheric modeling of upper troposphere and lower stratosphere, should be dismissed.
Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis
Ibusuki,Takeuchi
, p. 93 - 102 (1994)
Titanium dioxide (Tio2) oxidized nitric oxide (NO) to nitric acid (HNO3) very rapidly under ultraviolet light illumination, but some NO was oxidized to nitrogen dioxide (NO2), while activated carbon (AC) adsorbed NO2 well. A mixture of TiO2 and AC was thus confirmed to be an excellent photoassisted catalyst for removal of low concentration (sub-ppm) NOx from air. Addition of 1-3 wt % of ferric oxide to the mixture could markedly increase the catalytic activity. Even though the catalytic activity gradually declined with the reaction time, it could be completely recovery only by washing the catalyst was removed.
Complete, reversible H+/Li+ ion exchange reaction between rhombohedral LiMO3 and perovskite-type HMO3 (M = Nb, Ta)
Thangadurai,Weppner
, p. 2417 - 2425 (2002)
We demonstrate for the first time the complete, reversible H+/Li+ ion exchange reaction between HMO3 and LiMO3 (M=Nb, Ta) using molten LiNO3 at 320°C for 5 days. HMO3 were prepared from LiMO3 by ion exchange reactions using dilute HNO3. The results reveal that both LiMO3 prepared by normal solid state reaction between Li2CO3 and M2O5, and by ion exchanged from HMO3 using molten LiNO3 are isostructural. LiMO3 prepared using HMO3 yields nearly uniformly sized crystallites in contrast to those prepared by conventional solid state synthesis. The present method is simple and inexpensive compared to other methods of preparation of high purity LiMO3 powders.
A pulsed laser photolysis-pulsed laser induced fluorescence study of the kinetics of the gas-phase reaction of OH with NO2
Hynes,D'Ottone,Campuzano-Jost,Bauer
, p. 10538 - 10543 (2001)
The three-body recombination of OH with NO2 plays a critical role in tropospheric and stratospheric chemistry. The reaction plays a particularly important role in tropospheric ozone formation in polluted environments, acting as a sink for NO
Absolute Rate Constants for the Reaction of OH with NO2 in N2 and He from 225 to 389 K
Anderson, Larry G.
, p. 2152 - 2155 (1980)
The temperature dependence of the rate of the reaction OH + NO2 + N2 --> HNO3 + N2 was investigated by using a discharge flow system for OH production and resonance fluorescence for its detection.The reaction was investigated at room temperature in He, and between 225 and 389 K in N2.The temperature dependence could be fit by the Arrhenius expression (1.6 +/- 0.4)E-31 exp<(1560 +/- 270)/1.987T> cm6 molecule-2 s-1 or preferably by (2.3 +/- 0.6)E-30(T/298)-2.9 cm6 molecule-2 s-1.Earlier data have been used to determine the temperature dependence of the high-pressure limiting rate constant for this reaction.Troe's simplified expression for calculating rate constants in the falloff region was used to compare the appropriateness of different limiting values for the description of the experimentally observed pressure dependence of the rate constant.This reevaluation of the rate data suggests more appropriate values for k0 and k for use in atmospheric modeling: k0 = 2.3E-30(T/298)-2.9 cm6 molecule-2 s-1 and k = 1.2E-11(T/298)-1.6 cm3 molecule-1 s-1.
Detection of autocatalytic decomposition behavior of energetic materials using APTAC
Wei,Rogers,Mannan
, p. 125 - 130 (2006)
Characterization of autocatalytic decomposition reactions is important for the safe handling and storage of energetic materials. Isothermal differential scanning calorimetry (DSC) has been widely used to detect autocatalytic decomposition of energetic materials. However, isothermal DSC tests are time consuming and the choice of experimental temperature is crucial. This paper shows that an automatic pressure tracking calorimeter (APTAC) can be a reliable and efficient screening tool for the identification of autocatalytic decomposition behavior of energetic materials. Hydroxylamine nitrate (HAN) is an important member of the hydroxylamine family. High concentrations of HAN are used as liquid propellants, and low concentrations of HAN are used primarily in the nuclear industry for decontamination of equipment. Because of its instability and autocatalytic decomposition behavior, HAN has been involved in several incidents. This paper presents calorimetric measurements for the thermal decomposition of 24 mass% HAN/water. APTAC heat-wait-search and heat-soak-search modes are used to characterize the thermal decomposition of HAN. By comparing the kinetic analysis for the two modes, it is concluded that HAN shows strong autocatalytic decomposition behavior. The most likely decomposition pathway of HAN is proposed to explain the observed autocatalytic behavior.
Reduction of NO2 to nitrous acid on illuminated titanium dioxide aerosol surfaces: Implications for photocatalysis and atmospheric chemistry
Gustafsson, R. Joel,Orlov, Alexander,Griffiths, Paul T.,Cox, R. Anthony,Lambert, Richard M.
, p. 3936 - 3938 (2006)
TiO2, a component of atmospheric mineral aerosol, catalyses the reduction of NO2 to nitrous acid (HONO) when present as an aerosol and illuminated with near UV light under conditions pertinent to the troposphere. The Royal Society of Chemistry 2006.
Facile synthesis of double cone-shaped Ag4V2O7/BiVO4 nanocomposites with enhanced visible light photocatalytic activity for environmental purification
Hu, Yang,Fan, Jun,Pu, Chenchen,Li, Hua,Liu, Enzhou,Hu, Xiaoyun
, p. 172 - 183 (2017)
Ag4V2O7/BiVO4 photocatalysts with double cone-shaped nanostructure were successfully synthesized by a facile sodium polyphosphate-assisted hydrothermal method. The results demonstrate that coupling Ag4V2O7 with BiVO4 can promote the separation of photoinduced charge carriers and enhance the photon absorption efficiency. Experimental results indicate that Ag4V2O7/BiVO4 composites exhibit the enhanced photocatalystic activity for degradation of methylene blue (MB) and oxidation of NO in high concentrate (1600?ppb) compared to the pure BiVO4 under visible light irradiation (λ?>?420?nm). The composite with 0.08 mol% Ag4V2O7 has the highest photocatalytic activity. MB degradation rate can reach 98.48% in 1?h and NO oxidation rate can reach 52.83% in 0.5?h on 0.08-Ag4V2O7/BiVO4, which are about 2.90 and 3.11 times higher than that of pure BiVO4 respectively. The excellent activity can be attributed to the efficient charge transfer between Ag4V2O7 and BiVO4, and active species h+ and [rad]O2? play important roles during MB degradation and NO oxidation. In addition, this composite exhibits favorable stability during the cycling experiment, suggesting it may be a promising visible light active photocatalyst for environmental applications.
Aqueous phase reactivity of nitrate radicals (NO3) toward dicarboxylic acids
De Sémainville,D'Anna,George
, p. 1247 - 1260 (2010)
Laser photolysis technique was used to study the reactivity of nitrate radical towards four dicarboxylic acids. The temperature dependence of the reactions was investigated in the range from 278K to 318K. The effect of the acid-base equilibrium was examined by measuring the activation parameters of the dissociated and undissociated acids at different pH. For droplets or aerosols under moderate acidic conditions, the charge exchange reaction at the carboxylate group might compete with the oxidation by the hydroxyl radical. On liquid particles enriched in nitrate (such as a deliquescent ammonium nitrate), the lifetime of carboxylic groups towards the NO3 radical may be as short as few hours (or less). by Oldenbourg Wissenschaftsverlag.
PLATINUM-ACRIDINE COMPOUNDS AND METHODS OF TREATING CANCERS
-
, (2021/10/30)
Platinum-acridines and analogs thereof as cytotoxic agents for cancer treatment. Also provided methods of using hMATE1 (SLC47A1) as a biomarker to identify tumors that are likely to respond to the agents, and epigenetically sensitizing tumor tissue to anticancer drugs targeting this membrane transporter.
Thermal behavior of ammonium dinitramide and amine nitrate mixtures
Matsunaga, Hiroki,Katoh, Katsumi,Habu, Hiroto,Noda, Masaru,Miyake, Atsumi
, p. 2677 - 2685 (2018/11/23)
This paper focuses on the thermal behavior of mixtures of ammonium dinitramide (ADN) and amine nitrates. Because some mixtures of ADN and amine nitrate exhibit low melting points and high-energy content, they represent potential liquid propellants for spacecraft. This study focused on the melting behavior and thermal-decomposition mechanisms in the condensed phase of ADN/amine nitrate mixtures during heating. We measured the melting point and exothermal behavior during constant-rate heating using differential scanning calorimetry and performed thermogravimetry–differential thermal analysis–mass spectrometry (TG–DTA–MS) to analyze the thermal behavior and evolved gases of ADN/amine nitrate mixtures during simultaneous heating to investigate their reaction mechanisms. Results showed that the melting point of ADN was significantly lowered upon the addition of amine nitrate with relatively low molecular volume and low melting point. TG–DTA–MS results showed that the onset temperature of the thermal decomposition of ADN/amine nitrates was similar to that of pure ADN. Furthermore, during thermal decomposition in the condensed phase, ADN produced highly acidic products that promoted exothermic reactions, and we observed the nitration and nitrosation of amines from the dissociation of amine nitrates.
Capture of nitrogen dioxide and conversion to nitric acid in a porous metal–organic framework
Li, Jiangnan,Han, Xue,Zhang, Xinran,Sheveleva, Alena M.,Cheng, Yongqiang,Tuna, Floriana,McInnes, Eric J. L.,McCormick McPherson, Laura J.,Teat, Simon J.,Daemen, Luke L.,Ramirez-Cuesta, Anibal J.,Schr?der, Martin,Yang, Sihai
, p. 1085 - 1090 (2019/11/29)
Air pollution by nitrogen oxides, NOx, is a major problem, and new capture and abatement technologies are urgently required. Here, we report a metal–organic framework (Manchester Framework Material 520 (MFM-520)) that can efficiently confine dimers of NO2, which results in a high adsorption capacity of 4.2 mmol g–1 (298 K, 0.01 bar) with full reversibility and no loss of capacity over 125 cycles. Treatment of NO2?MFM-520 with water in air leads to a quantitative conversion of the captured NO2 into HNO3, an important feedstock for fertilizer production, and fully regenerates MFM-520. The confinement of N2O4 inside nanopores was established at a molecular level, and the dynamic breakthrough experiments using both dry and humid NO2 gas streams verify the excellent stability and selectivity of MFM-520 and confirm its potential for precious-metal-free deNOx technologies.