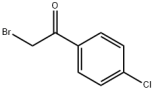
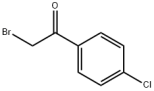
4-Chloro-2'-bromoacetophenone literature
Synthesis of novel thiazolo[2,3-b]quinazolines by cyclization reaction of octahydroquinazoline-2-thiones with α-bromoketones
Quan, Zheng-Jun,Wei, Ying,Wang, Xi-Cun
, p. 181 - 185 (2011)
Novel thiazolo[2,3-b]quinazolines were prepared by the cyclization reaction between octahydroquinazoline-2-thiones with α-bromoketones, which provides a readily accessible multifunctionalized quinazoline template for diversity-oriented synthesis. 2011 · Copyright by Walter de Gruyter.
Synthesis of a New Phorbazole and Its Derivatives
Louglin, Wendy A.,Muderawan, I Wayan,Young, David J.
, (2021/11/30)
Phorbazoles are chlorinated marine alkaloids containing pyrrole, oxazole and phenol ring units, and differ in the number and positions of chlorine atoms. They are isolated from sea sponges and nudibranchs. In this work, a convenient synthetic method leading to a new phorbazole and its derivatives is developed. This synthesis of synthetic phorbazole G and its derivatives is achieved in seven steps in good overall yields of 26-52%. It involves formation of the pyrrole-oxazole skeleton followed by chlorination. The pyrrole-oxazole skeleton is synthesized from pyrrole and substituted acetophenones, and the key step involves cyclodehydration of amide intermediates to give protected oxazoles, followed by hydrolysis.
A practical synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions
Wang, Zhihui,Wang, Lei,Wang, Zhiming,Li, Pinhua,Zhang, Yicheng
supporting information, p. 429 - 432 (2020/02/29)
A practical synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions has been developed. In the presence of PhI(OAc)2 as promoter and under ambient conditions, the reactions of styrenes and triiodomethane undergo the transformation smoothly to deliver the corresponding α-iodoketones without additional photocatalyst in good yields under sunlight irradiation. Meanwhile, the reactions of styrenes with tribromomethane and trichloromethane generate the desired α-bromoketones and α-chloroketones in high yields by using Ru(bpy)3Cl2 as a photocatalyst under blue LED (450–455 nm) irradiation.
Solvent-free preparation of α,α-dichloroketones with sulfuryl chloride
Tu, Dewei,Luo, Juan,Jiang, Wengao,Tang, Qiang
supporting information, (2021/09/15)
An efficient and facile method is reported for the synthesis of a series of α,α-dichloroketones. The direct dichlorination of methyl ketones and 1,3-dicarbonyls using an excess amount of sulfuryl chloride affords the corresponding gem-dichloro compounds in moderate to excellent yields. Moreover, the protocol features high yields, broad substrate scope, and simple reaction conditions without using any catalysts and solvents.
Thiazole ring-containing amide compounds as well as preparation method and application thereof
-
Paragraph 0044; 0051; 0063; 0066; 0129; 0134; 0213; 0218, (2021/06/23)
The invention discloses thiazole ring-containing amide compounds as well as a preparation method and application thereof, and belongs to the field of chemical technologies and pesticides. According to the present invention, p-phenylenediamine is adopted as a raw material to synthesize a series of the thiazole ring-containing amide compounds, and the synthesized thiazole ring-containing amide compounds have good inhibition effects on Xanthomonas oryzae pv.Oryza (Xoo), Xanthomonas oryzae pv.Oryzcola (Xoc) and Xanthomonas axonophora pv.Citri (Xac) in agricultural diseases and insect pests, and can be used for preparing the anti-plant bacterium agent.