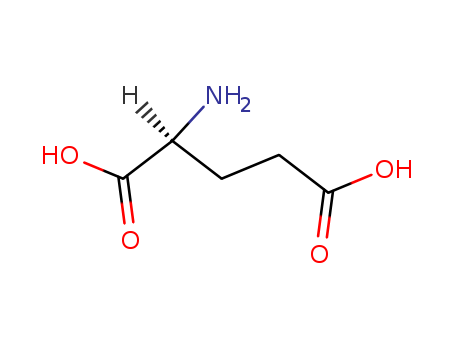
L-Glutamic acid (alpha) literature
Structures and antitumor activities of ten new and twenty known surfactins from the deep-sea bacterium Limimaricola sp. SCSIO 53532
Chen, Min,Chen, Rouwen,Ding, Wenping,Li, Yanqun,Tian, Xinpeng,Yin, Hao,Zhang, Si
, (2022/01/11)
Surfactins are natural biosurfactants with myriad potential applications in the areas of healthcare and environment. However, surfactins were almost exclusively produced by the bacterium Bacillus species in previous reported literatures, together with difficulty in isolating pure monomer, which resulted in making extensive effort to remove duplication and little discovery of new surfactins in recent years. In the present study, the result of Molecular Networking indicated that Limimaricola sp. SCSIO 53532 might well be a potential resource for surfacin-like compounds based on OSMAC strategy. To search for new surfactins with significant biological activity, further study was undertaken on the strain. As a result, ten new surfactins (1–10), along with twenty known surfactins (11–30), were isolated from the ethyl acetate extract of SCSIO 53532. Their chemical structures were established by detailed 1D and 2D NMR spectroscopy, HRESIMS data, secondary ion mass spectrometry (MS/MS) analysis, and chemical degradation (Marfey's method) analysis. Cytotoxic activities of twenty-seven compounds against five human tumor cell lines were tested, and five compounds showed significant antitumor activities with IC50 values less than 10 μM. Furtherly, analysis of structure–activity relationships revealed that the branch of side chain, the esterification of Glu or Asp residue, and the amino acid residue of position 7 possessed a great influence on antitumor activity.
Noncovalently Functionalized Commodity Polymers as Tailor-Made Additives for Stereoselective Crystallization
Wan, Xinhua,Wang, Zhaoxu,Ye, Xichong,Zhang, Jie
supporting information, p. 20243 - 20248 (2021/08/09)
Stereoselective inhibition of the nucleation and crystal growth of one enantiomer aided by “tailor-made” polymeric additives is an efficient method to obtain enantiopure compounds. However, the conventional preparation of polymeric additives from chiral monomers are laborious and limited in structures, which impedes their rapid optimization and applicability. Herein, we report a “plug-and-play” strategy to facilitate synthesis by using commercially available achiral polymers as the platform to attach various chiral small molecules as the recognition side-chains through non-covalent interactions. A library of supramolecular polymers made up of two vinyl polymers and six small molecules were applied with seeds in the selective crystallization of seven racemates in different solvents. They showed good to excellent stereoselectivity in yielding crystals with high enantiomeric purities in conglomerates and racemic compound forming systems. This convenient, low-cost modular synthesis strategy of polymeric additives will allow for high-efficient, economical resolution of various racemates on different scales.
Isolation, Structure Determination, and Total Synthesis of Hoshinoamide C, an Antiparasitic Lipopeptide from the Marine Cyanobacterium Caldora penicillata
Iwasaki, Arihiro,Ohtomo, Keisuke,Kurisawa, Naoaki,Shiota, Ikuma,Rahmawati, Yulia,Jeelani, Ghulam,Nozaki, Tomoyoshi,Suenaga, Kiyotake
, p. 126 - 135 (2021/01/13)
Hoshinoamide C (1), an antiparasitic lipopeptide, was isolated from the marine cyanobacterium Caldora penicillata. Its planar structure was elucidated by spectral analyses, mainly 2D NMR, and the absolute configurations of the α-amino acid moieties were determined by degradation reactions followed by chiral-phase HPLC analyses. To clarify the absolute configuration of an unusual amino acid moiety, we synthesized two possible diastereomers of hoshinoamide C and determined its absolute configuration based on a comparison of their spectroscopic data with those of the natural compound. Hoshinoamide C (1) did not exhibit any cytotoxicity against HeLa or HL60 cells at 10 μM, but inhibited the growth of the parasites responsible for malaria (IC50 0.96 μM) and African sleeping sickness (IC50 2.9 μM).
Leveraging Peptaibol Biosynthetic Promiscuity for Next-Generation Antiplasmodial Therapeutics
Lee, Jin Woo,Collins, Jennifer E.,Wendt, Karen L.,Chakrabarti, Debopam,Cichewicz, Robert H.
supporting information, p. 503 - 517 (2021/03/01)
Malaria remains a worldwide threat, afflicting over 200 million people each year. The emergence of drug resistance against existing therapeutics threatens to destabilize global efforts aimed at controlling Plasmodium spp. parasites, which is expected to leave vast portions of humanity unprotected against the disease. To address this need, systematic testing of a fungal natural product extract library assembled through the University of Oklahoma Citizen Science Soil Collection Program has generated an initial set of bioactive extracts that exhibit potent antiplasmodial activity (EC50 < 0.30 μg/mL) and low levels of toxicity against human cells (less than 50% reduction in HepG2 growth at 25 μg/mL). Analysis of the two top-performing extracts from Trichoderma sp. and Hypocrea sp. isolates revealed both contained chemically diverse assemblages of putative peptaibol-like compounds that were responsible for their antiplasmodial actions. Purification and structure determination efforts yielded 30 new peptaibols and lipopeptaibols (1-14 and 28-43), along with 22 known metabolites (15-27 and 44-52). While several compounds displayed promising activity profiles, one of the new metabolites, harzianin NPDG I (14), stood out from the others due to its noteworthy potency (EC50 = 0.10 μM against multi-drug-resistant P. falciparum line Dd2) and absence of gross toxicity toward HepG2 at the highest concentrations tested (HepG2 EC50 > 25 μM, selectivity index > 250). The unique chemodiversity afforded by these fungal isolates serves to unlock new opportunities for translating peptaibols into a bioactive scaffold worthy of further development.