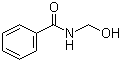
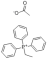
N-(Hydroxymethyl)benzamide literature
Preparation method of 4AA
-
Paragraph 0016; 0018; 0021; 0024, (2021/07/28)
The invention discloses a preparation method of 4AA. The preparation method comprises the following steps: S1, preparing a first intermediate from benzamide and a formaldehyde aqueous solution; S2, preparing a second intermediate from the first intermediate, thionyl chloride, toluene and n-heptane; S3, preparing a third intermediate from the second intermediate, methyl acetoacetate, sodium methoxide, toluene, diluted hydrochloric acid and isopropanol; S4, preparing a fourth intermediate from the third intermediate, reductase, ethyl acetate, saturated sodium bicarbonate and saturated salt water; S5, preparing a fifth intermediate from the fourth intermediate, imidazole, TBSCL and methylbenzene; S6, preparing a sixth intermediate from the fifth intermediate, ethanolamine, methanol and n-heptane; S7, preparing a seventh intermediate by using the sixth intermediate, a Grignard reagent and n-heptane; and S8, preparing 4AA from the seventh intermediate, ruthenium trichloride, potassium acetate, ethyl acetate, acetic acid and a peracetic acid solution.
Three-component synthesis of amidomethylarenes and -heteroarenes over HΒ zeolite under solvent-free conditions
Chevella, Durgaiah,Mameda, Naresh,Kodumuri, Srujana,Banothu, Rammurthy,Gajula, Krishna Sai,Kutepov, Boris Ivanovich,Nama, Narender
, p. 20 - 25 (2017/11/20)
A highly efficient and eco-friendly protocol has been described for the synthesis of amidomethylarenes and -heteroarenes via one-pot three-component coupling reaction of amides, aldehydes and (hetero)arenes over a heterogeneous catalyst (Hβ zeolite) in solvent-free conditions. The scope and limitations of this catalytic process are demonstrated with various amides and arenes and the corresponding amidoalkylated arene products were obtained in moderate to excellent yields. The preliminary mechanistic insight (control experiments) suggest that bisamide and/or N-(hydroxymethyl)benzamide are probable intermediates in this reaction. Moreover, the catalyst can be reused without any significant loss of the catalytic activity and only water is produced as by-product.
A convenient and clean synthesis of methylenebisamides and carbinolamides over zeolites in aqueous media
Mameda, Naresh,Marri, Mahender Reddy,Peraka, Swamy,Macharla, Arun Kumar,Kodumuri, Srujana,Chevella, Durgaiah,Naresh, Gutta,Nama, Narender
, p. 41 - 43 (2015/02/02)
A simple, efficient and environmentally benign protocol for the synthesis of methylenebisamides and carbinolamides in high yields from aromatic amides and formaldehyde in the presence of heterogeneous catalysts (Hβ and NaY zeolites) using water as a solvent is demonstrated. Moreover, the catalyst is recyclable and can be reused without significant loss in its catalytic activity.
Bi(OTf)3-Catalyzed Multicomponent α-Amidoalkylation Reactions
Schneider, Angelika E.,Manolikakes, Georg
, p. 6193 - 6212 (2015/06/30)
A bismuth(III) triflate catalyzed three-component synthesis of α-substituted amides starting from amides, aldehydes, and (hetero)arenes is reported. The reaction has a broad substrate scope, encompassing formaldehyde as well as aryl and alkyl aldehydes. Low catalyst loadings are required, and water is formed as the only side product. The scope and limitation of this method will be discussed.