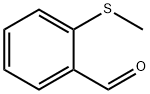
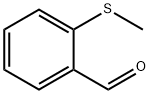
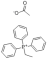
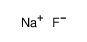2-(METHYLTHIO) BENZALDEHYDE literature
Rhodium(III)-Catalyzed Aldehyde C?H Activation and Functionalization with Dioxazolones: An Entry to Imide Synthesis
Bellière-Baca, Virginie,Clavier, Hervé,Hérault, Damien,Massouh, Joe,Petrelli, Antoine
supporting information, (2022/01/06)
A rhodium(III)-based catalytic system has been used to develop a C?H bond activation of benzaldehyde derivatives and subsequent functionalization with dioxazolones in order to afford imides. The importance of the nature of the directing group to perform selectively the aldehydic C?H bond activation has been highlighted. The scope investigation showed that this transformation could be applied to various dioxazolones and many benzaldehyde derivatives as well as an acrolein derivative. Derivatization reactions of the imide products demonstrated the synthetic utility of this rhodium-catalyzed aldehydic C?H amidation.
Nickel-Catalyzed Inter- and Intramolecular Aryl Thioether Metathesis by Reversible Arylation
Delcaillau, Tristan,Bismuto, Alessandro,Lian, Zhong,Morandi, Bill
supporting information, p. 2110 - 2114 (2019/12/24)
A nickel-catalyzed aryl thioether metathesis has been developed to access high-value thioethers. 1,2-Bis(dicyclohexylphosphino)ethane (dcype) is essential to promote this highly functional-group-tolerant reaction. Furthermore, synthetically challenging macrocycles could be obtained in good yield in an unusual example of ring-closing metathesis that does not involve alkene bonds. In-depth organometallic studies support a reversible Ni0/NiII pathway to product formation. Overall, this work not only provides a more sustainable alternative to previous catalytic systems based on Pd, but also presents new applications and mechanistic information that are highly relevant to the further development and application of unusual single-bond metathesis reactions.
Unexpected formation of 4,7-dihalobenzo[b]thiophenes using Ohira-Bestmann reagent and reactivity of the halogen-substituted benzo[b]thiophenes in Suzuki-Miyaura coupling with phenylboronic acid
Toyota, Kozo,Mutoh, Hirotaka,Kishi, Hiroki,Mikami, Shinichi,Tanaka, Hiroki,Yoshida, Shuhei,Naganuma, Daisuke
, p. 1355 - 1374 (2019/12/23)
Reaction of 2-(1-adamantylsulfanyl)-3,6-dihalobenzaldehydes with Ohira-Bestmann reagent gave 4,7-dihalobenzo[b]thiophenes along with normal alkyne products. Nine types of 4,7-dihalobenzo[b]thiophenes bearing chlorine, bromine, or iodine atoms, were prepared by this method. Regioselectivity in Suzuki-Miyaura cross coupling reactions of the 4,7-dihalobenzo[b]thiophenes with PhB(OH)2 was also studied.
Construction of highly functionalized thiophene and benzo[b]thiophene derivatives via a sequence of propargyl–allenyl isomerization/cyclization/demethylation
Chen, Dianpeng,Xing, Gangdong,Chen, Xueyuan,Yao, Jinzhong,Zhou, Hongwei
supporting information, p. 5124 - 5126 (2016/11/09)
An efficient one-pot protocol for the synthesis of functionalized thiophene and benzo[b]thiophene derivatives was developed via a sequence of propargyl–allenyl isomerization/cyclization/demethylation. As a result of the readily accessible starting materials, simple operation, and mild conditions, this reaction should have potential utility in organic synthesis.