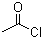
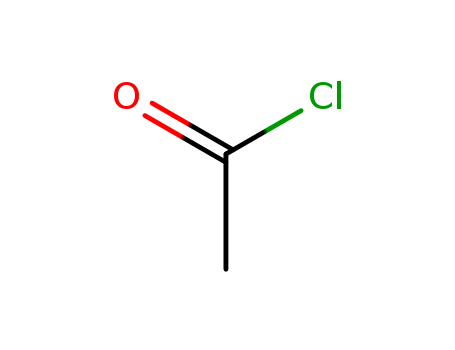
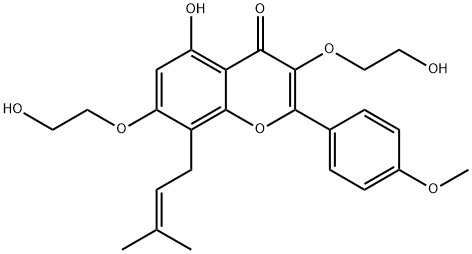
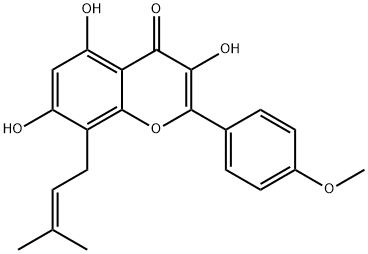
Acetyl chloride literature
Photopolymerization of Disordered Solid Acetaldehyde at Crygenic Temperature
Mansueto, Edward S.,Wight, Charles A.
, p. 1502 - 1504 (1992)
Binary solid solutions of 5percent chlorine in acetaldehyde were formed by vapor deposition onto the surface of a CsI optical window at 77 and 10 K.Laser photolysis of the low-temperature films at 308 nm induces a chemical reaction forming acetyl chloride and oligomeric acetaldehyde as the major products.The average chain length of the oligomer is 8.1 +/- 1.1 monomer units for the 77 K experiments.Films deposited and photolyzed at 10 K exhibit smaller chain lengths which grow upon warming to temperature above 40 K.The photochemical behavior of this system parallels that of well-studied formaldehyde polymerization reaction in the solid state in which the principal barriers to chain propagation are spatial and orientational discordering of molecules in the solid lattice.
Chemical decarbonylation of organometallic compounds
Alexander, John J.,Wojcicki, Andrew
, p. 74 - 76 (1973)
Transition metal carbonyl acyl complexes of the type π-C5H5M(CO)nCOR (M = Fe, n = 2; M, = Mo, n = 3) can be chemically decarbonylated under mild conditions using Rh[P(C6H5)3]3Cl. The products are a mixture of the corresponding alkyl and triphenylphosphine-substituted acyl complexes. The scope and mechanism of this reaction are discussed.
1,1-Dichloroethyl Hydroperoxide and 1,1-Dichloroethyl Peroxide Ion as Intermediates in the Ozonolysis of 2,3-Dichloro-2-butene
Gaeb, Siegmar,Turner, Walter V.
, p. 2711 - 2714 (1984)
On the basis of its spectroscopic and chemical properties, an unstable intermediate previously detected in the ozonolysis of trans-2,3-dichloro-2-butene (1) in inert solvents is reformulated as 1,1-dichloroethyl hydroperoxide (5).Ozonolysis of 1 in ethyl formate saturated with anhydrous HCl leads to high yields of this intermediate. 1,1-Dichloroethyl peroxide ion (10), rather then 5, is believed to be the precursor of acetyl 1,1-dichloroethyl peroxide (8), which is produced in higher yield on ozonolysis of 1 in the presence of tetraalkylammonium chloride.
-
Porter,Iloff
, p. 6200 (1974)
-
Rhodium(III)-catalyzed chemodivergent annulations between phenyloxazoles and diazos via C–H activation
Zhang, Xueguo,Wang, Peigen,Zhu, Liangwei,Chen, Baohua
supporting information, p. 695 - 699 (2020/06/28)
Acid-controlled, chemodivergent and redox-neutral annulations for the synthesis of isocoumarins and isoquinolinones have been realized via Rh(III)-catalyzed C[sbnd]H activation. Diazo compounds act as a carbene precursor, and coupling occurs in one-pot process, where adipic acid and trimethylacetic acid promote chemodivergent cyclizations.
Benzimidazole tethered thioureas as a new entry to elastase inhibition and free radical scavenging: Synthesis, molecular docking, and enzyme inhibitory kinetics
Abbas, Qamar,Ashraf, Saba,Channar, Pervaiz Ali,Hassan, Abbas,Hassan, Mubashar,Rafique, Hummera,Raza, Hussain,Rind, Mahboob Ali,Saeed, Aamer,Seo, Sung-Yum,Ujan, Rabail,Ul-Hamid, Anwar
, (2021/07/02)
The porcine pancreatic elastase inhibition and free-radical scavenging play a crucial role in age progression. All the series of 10 newly synthesized benzimidazole thioureas (4a-j) were assessed for elastase inhibition and radical scavenging activity to identify the suitable anti-aging ingredient for cosmetics products. The compounds 4e, 4f, 4g, and 4h showed inhibition better than the standard, while compound 4f showed the most significant elastase inhibition with the IC50 value of 1.318 ± 0.025 μM compared with oleanic acid IC50 13.451 ± 0.014 used ±1.989 and 41.563 ± 0.824, respectively, as standard. Molecular docking studies were performed and the compound 4f showed binding energy of 7.2 kcal/mol. Kinetics studies revealed inhibition of the pancreatic elastase in a competitive manner. The relative binding energy and structure activity relationship (SAR) identified compound 4f as an effective inhibitor of porcine pancreatic elastase. Compounds 4e and 4i showed remarkable free-radical scavenging activity with SC50 values of 26.421.
Neutrophil-Selective Fluorescent Probe Development through Metabolism-Oriented Live-Cell Distinction
Gao, Min,Lee, Sun Hyeok,Park, Sang Hyuk,Ciaramicoli, Larissa Miasiro,Kwon, Haw-Young,Cho, Heewon,Jeong, Joseph,Chang, Young-Tae
supporting information, p. 23743 - 23749 (2021/10/14)
Human neutrophils are the most abundant leukocytes and have been considered as the first line of defence in the innate immune system. Selective imaging of live neutrophils will facilitate the in situ study of neutrophils in infection or inflammation events as well as clinical diagnosis. However, small-molecule-based probes for the discrimination of live neutrophils among different granulocytes in human blood have yet to be reported. Herein, we report the first fluorescent probe NeutropG for the specific distinction and imaging of active neutrophils. The selective staining mechanism of NeutropG is elucidated as metabolism-oriented live-cell distinction (MOLD) through lipid droplet biogenesis with the help of ACSL and DGAT. Finally, NeutropG is applied to accurately quantify neutrophil levels in fresh blood samples by showing a high correlation with the current clinical method.
Design and synthesis of novel N-[3-(benzimidazol-2-ylamino)phenyl]amine and N-[3-(benzoxazol-2-ylamino)phenyl]amine derivatives as potential anticancer agents
Kumar, Honnavalli Yogish,Murumkar, Prashant R.,Pawar, Vijay,Srinivasan, B. P.,Yadav, M. R.
, (2021/10/20)
In this contribution, we report the design, synthesis and cytotoxicity studies of a series of N-[3-(benzimidazol-2-yl-amino)phenyl]amine and N-[3-(benzoxazol-2-ylamino)phenyl]amine derivatives. In vitro cytotoxicity assay of 26 selected compounds was carried out at National Cancer Institute (NCI), USA. Out of them, compounds 10e (NSC D-762842/1) and 11s (NSC D-764942/1) have shown remarkable cytotoxicity with GI50 values ranging between “0.589–14.3?μM” and “0.276–12.3?μM,” respectively, in the representative nine subpanels of human tumor cell lines. Further, flow cytometry analysis demonstrated that compound 10e exerted cell cycle arrest at G2/M phase and showed dose-dependent enhancement in apoptosis in K-562 leukemia cancer cells.