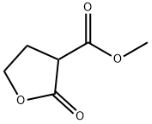
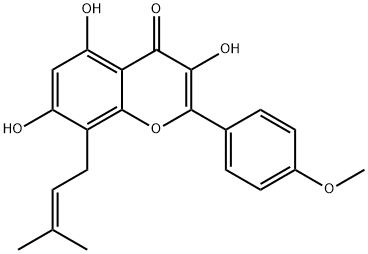
Colchicine literature
Gram-Scale, Seven-Step Total Synthesis of (-)-Colchicine
Liang, Xiao,Li, Lei,Wei, Kun,Yang, Yu-Rong
supporting information, p. 2731 - 2735 (2021/04/12)
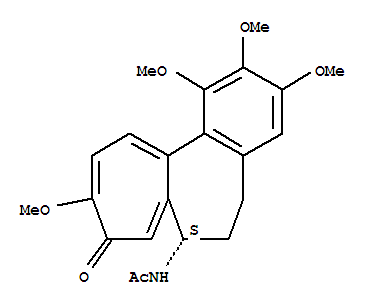
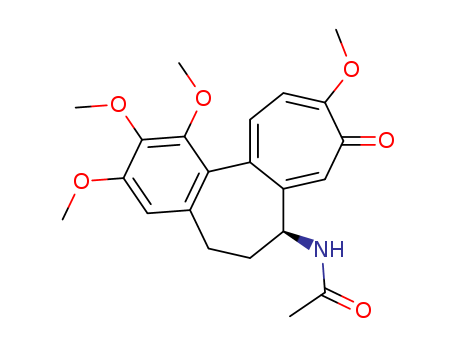
Herein we report a streamlined, gram-scale total synthesis of (-)-colchicine that takes only 7 easy steps, with an overall yield of 27-36%. To warrant the synthetic efficiency and practicality of (-)-colchicine, we tactically utilized a modified version of a powerful Ir-catalyzed amidation reported by Carreira to install the key chiral C-7 acetamido group, Suzuki and biomimetic phenol oxidative coupling, and Banwell-inspired cyclopropane ring cleavage to construct (-)-colchicine precisely and rapidly. Remarkably, a described strategy also can shorten the synthesis of allocolchicinoid to 4 steps.
SEMISYNTHETIC PROCESS FOR THE PREPARATION OF COLCHICINE
-
Page/Page column 11; 12, (2022/01/05)
The invention relates to a process for the preparation of colchicine 1 from colchicoside 2 which comprises enzymatic conversion of colchicoside 2 to 3-O- demethylcolchicine 3, wherein the enzyme used is a cellulase. According to another aspect of the invention, 3-O-demethylcolchicine 3 can be converted to colchicine 1 using an alkylating agent. The invention also relates to a process for enriching the colchicine 1 content of extracts from plants belonging to the Colchicaceae family containing colchicine 1, colchicoside 2 and 3 -(9-demethyl colchicine 3, which comprises conversion by means of a colchicoside 2 cellulase to 3-O-demethylcolchicine 3, followed by conversion of 3-O-demethylcolchicine 3 to colchicine 1 using an alkylating agent.
Asymmetric synthesis method of colchicine and allocolchicine
-
Paragraph 0040; 0043; 0058; 0066-0067, (2020/04/17)
The invention provides an asymmetric synthesis method of colchicine and allocolchicine, and belongs to the field of chemical synthesis. According to the invention, cheap commercial isovanillin A is used as a raw material, asymmetric allyl amination catalyzed by metal Ir is taken as a key reaction, a cyclization precursor E is obtained through Suzuki coupling reaction with a halide D, allocolchicine F is rapidly synthesized through intramolecular oxidative coupling, and finally, efficient asymmetric synthesis of colchicine I is completed through a bionic cyclopropane ring-opening strategy. Thesynthesis strategy used in the invention is simple and economic, good in operability and short in time consumption, and can meet the requirements of new drug development and large-scale preparation.
Asymmetric Total Syntheses of Colchicine, β-Lumicolchicine, and Allocolchicinoid N-Acetylcolchinol-O-methyl Ether (NCME)
Liu, Xin,Hu, Ya-Jian,Chen, Bo,Min, Long,Peng, Xiao-Shui,Zhao, Jing,Li, Shaoping,Wong, Henry N. C.,Li, Chuang-Chuang
supporting information, p. 4612 - 4615 (2017/09/12)
A concise and highly enantioselective synthesis of colchicine (>99% ee) in eight steps and 9.3% overall yield, without the need for protecting groups, was developed. A unique Wacker oxidation was used for enabling regioselective construction of the highly oxidized and synthetic challenging tropolone C-ring. Furthermore, asymmetric syntheses of β-lumicolchicine and N-acetylcolchinol-O-methyl ether (NCME) were achieved. Notably, NCME was synthesized from β-lumicolchicine by an unusual decarbonylation and electrocyclic ring-opening cascade reaction.