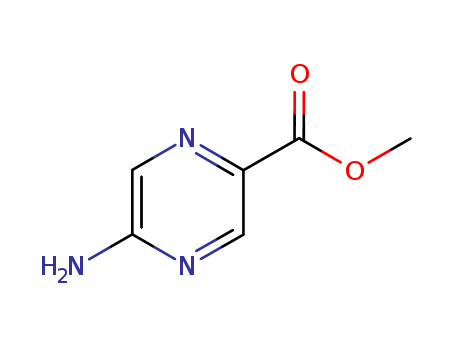
5-Aminopyrazine-2-carboxylic acid methyl ester literature
Design, synthesis and biological screening of 2-aminobenzamides as selective HDAC3 inhibitors with promising anticancer effects
Trivedi, Prakruti,Adhikari, Nilanjan,Amin, Sk. Abdul,Jha, Tarun,Ghosh, Balaram
, p. 165 - 181 (2018/09/12)
Histone deacetylases (HDACs) have been found as a potential target for anticancer therapy. A number of HDAC inhibitors have been used pre-clinically and clinically as anticancer agents. In the current study, we have designed and synthesized compound 12a by combining the scaffolds of CI-994 and BG45. Moreover, the structure of compound 12a was optimized and a series of 2-aminobenzamide derivatives were synthesized further. These compounds were tested for their HDAC inhibitory activity and found to be efficient HDAC inhibitors. Compound 26c showed 11.68-fold HDAC3 selectivity over pan HDACs, better than the prototype HDAC3 inhibitor BG45. Most of these compounds exhibited antiproliferative activity in both B16F10 and HeLa cell lines. Particularly, compound 26c exhibited better antitumor efficacy in the cell lines compared to the prototype inhibitors CI-994 and BG45. It was also found to promote apoptosis as well as induced significant cell growth arrest in the G2/M phase of cell cycle in B16F10 melanoma cells. This work may provide significant insight regarding structural information to design newer small molecule HDAC3 inhibitors to fight against the target specific malignancies in future.
Identification of RO4597014, a glucokinase activator studied in the clinic for the treatment of type 2 diabetes
Qian, Yimin,Corbett, Wendy L.,Berthel, Steven J.,Choi, Duk Soon,Dvorozniak, Mark T.,Geng, Wanping,Gillespie, Paul,Guertin, Kevin R.,Haynes, Nancy-Ellen,Kester, Robert F.,Mennona, Francis A.,Moore, David,Racha, Jagdish,Radinov, Roumen,Sarabu, Ramakanth,Scott, Nathan R.,Grimsby, Joseph,Mallalieu, Navita L.
supporting information, p. 414 - 418 (2013/07/25)
To resolve the metabolite redox cycling associated with our earlier clinical compound 2, we carried out lead optimization of lead molecule 1. Compound 4 showed improved lipophilic ligand efficiency and demonstrated robust glucose lowering in diet-induced obese mice without a liability in predictive preclinical drug safety studies. Thus, it was selected as a clinical candidate and further studied in type 2 diabetic patients. Clinical data suggests no evidence of metabolite cycling, which is consistent with the preclinical profiling of metabolism.
Highly efficient one-pot amination of carboxylate-substituted nitrogen-containing heteroaryl chlorides via Staudinger reaction
Kandalkar, Sachin R.,Kaduskar, Rahul D.,Ramaiah, Parimi Atchuta,Barawkar, Dinesh A.,Bhuniya, Debnath,Deshpande, Anil M.
supporting information, p. 414 - 418 (2013/02/23)
An efficient one-pot method for the synthesis of tert-butyl 6-aminonicotinate (5) is described. The key transformation involves displacement of the chloro group in tert-butyl 6-chloronicotinate (2) with azide followed by a Staudinger reaction. The scope of this methodology is further extended for the synthesis of a series of carboxylate-substituted heteroaryl amines. In particular, we synthesized tert-butyl carboxylate-substituted amino-pyridine, -pyridazine, and -pyrazine. In addition to one-pot conversion, short reaction time, simplicity of operation, ease of purification, and good yields are the key advantages of this methodology.
FUSED HETEROCYCLYC INHIBITOR COMPOUNDS
-
Page/Page column 124-125, (2010/03/02)
The present invention provides a compound of general Formula (I) having histone deacetylase (HDAC) and/or Cyclin-dependent kinase (CDK) inhibitory activity, a pharmaceutical composition comprising the compound, and a method useful to treat diseases using the compound