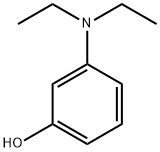
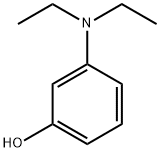
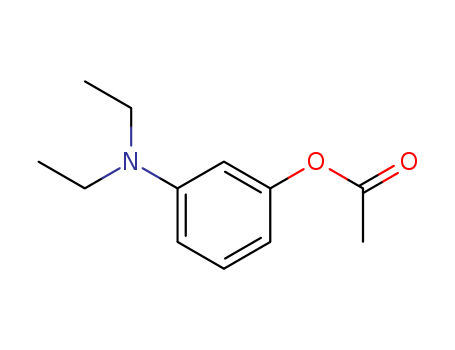
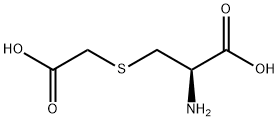
3-Diethylaminophenol literature
Mechanism study on Raney nickel-catalyzed amination of resorcinol
Ge, Xin,Pan, Jiong-Bin,Qian, Chao,Feng, Lie,Chen, Yun-Bin,Chen, Xin-Zhi
, p. 201 - 207 (2014)
Amination of resorcinol catalyzed by Raney nickel has been examined with good yield. Using the first principle density functional theory, some detailed mechanism of the amination of resorcinol on the Ni(111) surface is explored. The resorcinol is adsorbed on the Ni surface at the hollow site to form ketone by isomerization. The isomerization has a barrier of 122.1 kJ/mol. Ketone can couple with secondary amine mediated by resorcinol to afford hemiaminal. For the formation of hemiaminal, the steric effect of the alkyl group of secondary amine is obvious. Hemiaminal undergoes dehydration to get final product, which occurs by the preferred adsorption in the bridge site, cleavage of CO bond initially, followed by subsequent cleavage of CH bond.
Method for synthesizing M-diethylaminophenol
-
Paragraph 0016-0020, (2021/01/15)
The invention provides a method for synthesizing m-diethylaminophenol. The method comprises the following steps: mixing potassium hydroxide and magnesium oxide, and calcining at 700-800 DEG C to obtain a solid super base catalyst; adding sodium amide and the solid super base catalyst into diethylamine, stirring, keeping the temperature at 3-8 DEG C, dropwise adding o-chlorophenol within 20 minutes, stirring for 10-20 minutes after dropwise adding is finished, starting ultrasonic oscillation, heating, carrying out reflux reaction for 10-30 minutes, carrying out reduced pressure rotary evaporation to recover diethylamine, adding xylene, stirring, cooling to 5 DEG C or below, and adding water to quench the catalyst; and adjusting the pH value of a water layer to be neutral or weakly acidic, and recovering xylene to obtain the m-diethylaminophenol finished product. The method disclosed by the invention not only is short in time and high in efficiency, but also does not use expensive m-aminophenol raw materials, so that the problem of troublesome post-treatment is solved; meanwhile, potential safety hazards caused by using unsafe catalysts can be avoided, so that m-diethylaminophenol production is safer, more economical and more efficient, and industrialization is better facilitated.
Inter-alkane amidogen phenolic synthetic method (by machine translation)
-
Paragraph 0020, (2019/01/23)
Inter-alkane amidogen phenolic synthetic method, its characteristic is: 1st step, between the two alkane amidogen acyl aniline and sulfuric acid aqueous solution mixing and heating to 50 - 110 °C, thermal insulation reaction of aniline [...] sulfuric acid aqueous solution; 2nd step, continue to drip the sodium nitrite aqueous solution, sodium nitrite aqueous solution for dropping temperature of - 10 - 20 °C, drop bi yu 5 - 30 °C insulation, [...] aniline obtained diazonium salt of the sulfuric acid aqueous solution; 3rd step, [...] aniline diazonium salt of the sulfuric acid aqueous solution is directly heated to 45 - 110 °C, thermal insulation, in the hydrolysis reaction of the diazonium salt, cooling after treatment, to obtain the product between two alkane amidogen phenol; three-step required by the reaction of sulfuric acid in the 1st step reaction in the finished disposable adding; a three-step reaction in a finish step by step in the pot. The method of the invention with raw materials are cheap, abundant, synthetic high security of the process, the product yield is high, the three waste less pollution and the like, has high industrial value. (by machine translation)
Method for continuously synthesizing meta-di-alkane aminophenol
-
Paragraph 0020; 0032, (2019/01/23)
The invention discloses a method for continuously synthesizing meta-di-alkane aminophenol. The technical scheme includes that continuous diazotization or continuous diazonium salt hydrolysis or continuous diazotization and diazonium salt hydrolysis is carried on raw materials such as di-alkane aminophenol, sodium nitrite and sulfuric acid, and after-treatment such as extraction is carried out to obtain the meta-di-alkane aminophenol which is a product. The method has the advantages that the raw materials can come from sufficient sources and are low in cost, processes for synthesizing the meta-di-alkane aminophenol are high in safety, products are high in yield, the method is little in waste gas, wastewater and industrial residue pollution, is green and environmental friendly and has a highindustrialization value, and the like.
Synthesis process of sulfonic-group rhodamine compound
-
Paragraph 0027; 0030; 0033; 0035; 0038; 0040, (2019/04/26)
The invention discloses a synthesis process of a sulfonic-group rhodamine compound. The process comprises the steps: mixing saccharin and a protonic acid catalyst, performing heating for a reaction soas to obtain a compound shown in a formula (I), performing a reaction between a compound shown in a formula (II) and resorcinol through heating under the action of the protonic acid catalyst so as toobtain a compound shown in a formula (III), performing a reaction between the compound of the formula (I) and the compound in the formula (III) through heating under the action of a Lewis acid catalyst under the conditions of nitrogen protection and light shielding so as to obtain the sulfonic-group rhodamine compound shown in a formula (IV). Through the synthesis process, the use of thionyl chloride in a conventional process is avoided, the operation is simplified, the production safety is improved, the pollution to the environment is little, the reaction can be carried out under normal pressure, and the reaction has a high selectivity; and the chemical structural formulas of the compounds represented separately by the formula (I), the formula (II), the formula (III) and the formula (IV)are shown.