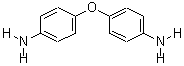
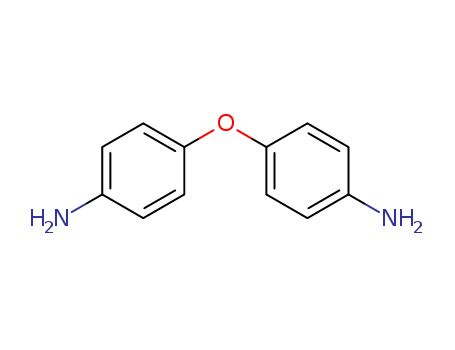
4,4'-Oxydianiline literature
Co-based heterogeneous catalysts from well-defined Α-diimine complexes: Discussing the role of nitrogen
Formenti, Dario,Ferretti, Francesco,Topf, Christoph,Surkus, Annette-Enrica,Pohl, Marga-Martina,Radnik, J?rg,Schneider, Matthias,Junge, Kathrin,Beller, Matthias,Ragaini, Fabio
, p. 79 - 89 (2017)
Ar-BIANs and related α-diimine Co complexes were wet impregnated onto Vulcan XC 72 R carbon black powder and used as precursors for the synthesis of heterogeneous supported nanoscale catalysts by pyrolysis under argon at 800?°C. The catalytic materials feature a core-shell structure composed of metallic Co and Co oxides decorated with nitrogen-doped graphitic layers (NGr). These catalysts display high activity in the liquid phase hydrogenation of aromatic nitro compounds (110?°C, 50 bar H2) to give chemoselectively substituted aryl amines. The catalytic activity is closely related to the amount and type of nitrogen atoms in the final catalytic material, which suggests a heterolytic activation of dihydrogen.
Phosphinic Acid as a Bifunctional Reagent in the Catalytic Bamberger Rearrangement of Nitrobenzene to para-Aminophenol
Zoran, Ami,Khodzhaev, Oleg,Sasson, Yoel
, p. 2239 - 2240 (1994)
Phosphinic acid is proposed as a simultaneous hydrogen donor and proton source in the Pd/C catalysed transformation of nitrobenzene to para-aminophenol.
Cocatalyst-Free Reduction of 4,4′-Dinitrodiphenyl Ether to 4,4′-Diaminodiphenyl Ether Over Twin-Crystal ZnxCd1?xS under Visible Light
Hu, Yujia,Yu, Guiyang,Xing, Chuanwang,Liu, Shanshan,Wei, Chuangyu,Liu, Heyuan,Jiang, Jianzhuang,Li, Xiyou
, p. 4591 - 4601 (2021)
Semiconductor-based photocatalytic conversion of solar energy is a promising method for the synthesis of high value-added chemicals. In this paper, a cocatalyst-free nano-twin crystal ZnxCd1?xS (T?ZnxCd1?xS) semiconductor was employed to achieve almost complete conversion of DNDPE and the yield of ODA product achieves >99 % in 40 min reaction time without additional hydrogen source. As far as we know, this is the first time to apply the photocatalytic technology for reducing DNDPE to ODA, and the photocatalytic efficiency has greatly exceeded the result of traditional catalytic method. Theoretical calculation and isotope labeling in situ HPLC-MS analysis demonstrates that the reduction mechanism of DNDPE is two nitro groups of DNDPE are separately instead of simultaneously reduced, following the process of DNDPE→NO2?C6H4?O?C6H4?NO→NO2?C6H4?O?C6H4?NHOH→NO2?C6H4?O?C6H4?NH2→NO?C6H4?O?C6H4?NH2→NH2?C6H4?O?C6H4?NHOH→ODA. Hydrogen protons of water, instead of ethanol, provide the hydrogen source for the photocatalytic reduction of DNDPE to ODA.
Biomass-Derived Catalysts for Selective Hydrogenation of Nitroarenes
Sahoo, Basudev,Formenti, Dario,Topf, Christoph,Bachmann, Stephan,Scalone, Michelangelo,Junge, Kathrin,Beller, Matthias
, p. 3035 - 3039 (2017)
Development of catalytically active materials from biowaste represents an important aspect of sustainable chemical research. Three heterogeneous materials were synthesized from inexpensive biomass-based chitosan and abundant Co(OAc)2 using complexation followed by pyrolysis at various temperatures. These materials were applied in the catalytic hydrogenation of nitroarenes using molecular hydrogen. A variety of diversely functionalized nitroarenes including some pharmaceutically active compounds were converted into aromatic amines in high yields, with high selectivity, and with excellent functional group tolerance. This green protocol has also been implemented for the synthesis of a biologically important TRPC3 inhibitor.
Synthesis method of 4, 4-amino diphenyl ether
-
Paragraph 0005; 0009; 0034-0045, (2020/12/30)
The invention discloses a synthesis method for synthesizing 4, 4-amino diphenyl ether in a water phase, and belongs to the field of organic synthesis. The method comprises the following steps: adding4-nitrochlorobenzene into water, adding potassium hydroxide and tetrabutylammonium bromide, and heating to react to obtain the 4, 4-nitrodiphenyl ether; and adding 4, 4-nitro diphenyl ether into water, adding hydrochloric acid and palladium on carbon, introducing hydrogen, and heating to react to obtain 4, 4-amino diphenyl ether. The method is easy and convenient to operate, no organic solvent isintroduced in the production process, the method is environmentally friendly, the yield of the obtained product is high, and the method is more suitable for large-scale production.
Mass production method of 4,4'-oxydianiline from 1,4-diiodobenzene
-
Paragraph 0024; 0026-0037, (2020/07/02)
The present invention relates to a method for producing 4,4andprime;-oxydianiline using 1,4-diiodobenzene. After 4,4andprime;-oxybis (iodobenzene) is produced by using a Cu catalyst and 1,4-diiodobenzene in a polar solvent mixed with water, 4,4andprime;-oxydianiline can be produced through amination with ammonia introduced without a separate purification process. Therefore, compared to the conventional invention, there is an advantage in that 4,4andprime;-oxydianiline can be directly produced through amination without an intermediate purification process. In addition, expensive iodine used in the reaction is recovered as ammonium iodide and can be used again to produce 1,4-diiodobenzene, so the process efficiency is high.
COPYRIGHT KIPO 2020
Synthetic method of 4, 4'-diaminodiphenyl ether
-
Paragraph 0013; 0034-0040, (2019/01/21)
The invention discloses a synthetic method of 4, 4'-diaminodiphenyl ether. The synthetic method comprises the following steps: S1, nitrosation reaction: adding sodium nitrite and diphenyl ether into awater phase, slowly dropwise adding hydrochloric acid in a range of 0-5 DEG C till the reaction is finished, separating out crystals, filtering the crystals, and drying the crystals in vacuum to obtain 4, 4'-diaminodiphenyl ether, wherein the reaction process is as follows; and S2, reductive reaction: adding the obtained solid into a high pressure kettle, adding a catalyst by taking alcohol as asolvent, pressurizing hydrogen to 0.5-4 MPa, heating and stirring the mixture to 70-120 DEG C, and keeping the temperature for 2-6 hours to obtain 4, 4'-diaminodiphenyl ether. The synthetic method disclosed by the invention can reduce the production cost, reduce the environmental pollution, improve the safety coefficient and reduce the emission of three wastes.