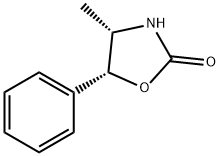
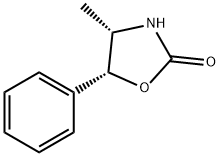
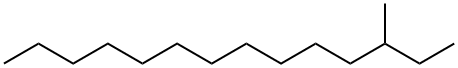
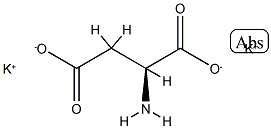
(4S,5R)-(-)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE literature
Enantioconvergent Cu-Catalyzed Radical C-N Coupling of Racemic Secondary Alkyl Halides to Access α-Chiral Primary Amines
Cheng, Jiang-Tao,Dong, Xiao-Yang,Gu, Qiang-Shuai,Li, Zhong-Liang,Liu, Juan,Liu, Xin-Yuan,Luan, Cheng,Wang, Fu-Li,Wang, Li-Lei,Yang, Ning-Yuan,Zhang, Yu-Feng
supporting information, p. 15413 - 15419 (2021/09/30)
α-Chiral alkyl primary amines are virtually universal synthetic precursors for all other α-chiral N-containing compounds ubiquitous in biological, pharmaceutical, and material sciences. The enantioselective amination of common alkyl halides with ammonia is appealing for potential rapid access to α-chiral primary amines, but has hitherto remained rare due to the multifaceted difficulties in using ammonia and the underdeveloped C(sp3)-N coupling. Here we demonstrate sulfoximines as excellent ammonia surrogates for enantioconvergent radical C-N coupling with diverse racemic secondary alkyl halides (>60 examples) by copper catalysis under mild thermal conditions. The reaction efficiently provides highly enantioenrichedN-alkyl sulfoximines (up to 99% yield and >99% ee) featuring secondary benzyl, propargyl, α-carbonyl alkyl, and α-cyano alkyl stereocenters. In addition, we have converted the masked α-chiral primary amines thus obtained to various synthetic building blocks, ligands, and drugs possessing α-chiral N-functionalities, such as carbamate, carboxylamide, secondary and tertiary amine, and oxazoline, with commonly seen α-substitution patterns. These results shine light on the potential of enantioconvergent radical cross-coupling as a general chiral carbon-heteroatom formation strategy.
Stereoselective synthesis of oxazolidin-2-ones via an asymmetric aldol/curtius reaction: Concise total synthesis of (?)-cytoxazone
Choi, Hosam,Choi, Joohee,Jang, Hanho,Lee, Kiyoun
, (2021/06/14)
Herein, we are reporting an efficient approach toward the synthesis of 4,5-disubstituted oxazolidin-2-one scaffolds. The developed approach is based on a combination of an asymmetric aldol and a modified Curtius protocol, which uses an effective intramolecular ring closure to rapidly access a range of oxazolidin-2-one building blocks. This strategy also permits a straightforward and concise asymmetric total synthesis of (?)-cytoxazone. Consisting of three steps, this is one of the shortest syntheses reported to date. Ultimately, this convenient platform would provide a promising method for the early phases of drug discovery.
Tandem copper and photoredox catalysis in photocatalytic alkene difunctionalization reactions
Reed, Nicholas L.,Herman, Madeline I.,Miltchev, Vladimir P.,Yoon, Tehshik P.
supporting information, p. 351 - 356 (2019/02/20)
Oxidative alkene difunctionalization reactions are important in synthetic organic chemistry because they can install polar functional groups onto simple non-polar alkene moieties. Many of the most common methods for these reactions rely upon the reactivity of pre-oxidized electrophilic heteroatom donors that can often be unstable, explosive, or difficult to handle. Herein, we describe a method for alkene oxyamination and diamination that utilizes simple carbamate and urea groups as nucleophilic heteroatom donors. This method uses a tandem copper–photoredox catalyst system that is operationally convenient. The identity of the terminal oxidant is critical in these studies. Ag(I) salts proved to be unique in their ability to turn over the copper cocatalyst without deleteriously impacting the reactivity of the organoradical intermediates.
A new type of L-Tertiary leucine-derived ligand: Synthesis and application in Cu(II)-catalyzed asymmetric Henry reactions
Cai, Zedong,Lan, Ting,Ma, Pengfei,Zhang, Jingfang,Yang, Qingqing,He, Wei
, (2019/08/08)
A new series of Schiff bases derived from amino acids were developed as chiral ligands for Cu(II)-catalyzed asymmetric Henry reactions. The optimum ligand 7d exhibited outstanding catalytic efficiency in the Cu(II)-catalyzed asymmetric Henry additions of four nitroalkanes to different kinds of aldehydes to produce 76 desired adducts in high yields (up to 96%) with excellent enantioselectivities, up to 99% enantiomeric excess (ee).