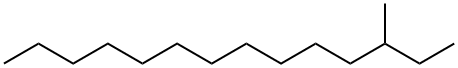
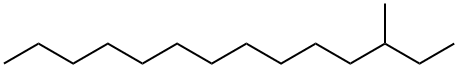
3-methyl tetradecane Appearance/Package/Shipping/Storage
Package
25kg/drum, 1kg/bottle, according to client's requirements
Storage condition
Store in cool, dry, and well ventilated place
3-methyl tetradecane literature
Process for hydrogenation of carboxylic acids and derivatives to hydrocarbons
-
Page/Page column 7-8, (2008/06/13)
A process for hydrogenating a carboxylic acid and/or derivative thereof having a carboxylate group represented by the general formula R1COO-, which process comprises feeding hydrogen and the carboxylic acid and/or derivative thereof to a reactor and maintaining conditions within the reactor such that hydrogen reacts with the carboxylic acid and/or derivative thereof to produce a product stream comprising carbon dioxide, carbon monoxide, methane and hydrocarbons represented by general formulae R1H and R1CH3, characterised in that the molar ratio of R1H : R1CH3 is above a pre-determined value and/or the mole ratio of the sum of carbon dioxide, carbon monoxide and methane to carboxylate groups is above a pre-determined value.
SELECTIVE MIXED COUPLING OF CARBOXYLIC ACIDS (I). - ELECTROLYSIS, THERMOLYSIS AND PHOTOLYSIS OF UNSYMMETRICAL DIACYL PEROXIDES WITH ACYCLIC AND CYCLIC ALKYL GROUPS
Feldhues, Michael,Schaefer, Hans J.
, p. 4195 - 4212 (2007/10/02)
14 unsymmetrical diacyl peroxides (R1CO2-O2CR2 with R1: undecyl; R2: e.g. methyl, propyl, pentyl, nonyl, 2-methylpropyl, 2-propyl, 2-pentyl, cyclopropyl, cyclobutyl, cyclohexyl) are prepared in 85 to 92 percent yield.Square pulse electrolysis of dodecanoyl octanoyl peroxide (1i) affords the unsymmetrical coupling product octadecane (4) in poor yield and selectivity.Thermolysis or photolysis in solution produces preferentially 4, but also considerable amounts of disproportionation products.At -78 deg C the neat peroxides are photolysed selectively to the mixed dimers.With straight chain and β-branched alkyl groups high yields are obtained (63 - 76 percent), with cycloalkyl groups medium yields (42 - 56 percent), and with α-branched diacyl peroxides moderate yields (20 - 33 percent).A comparison of the mixed Kolbe-electrolysis with the low temperature photolysis of the neat peroxide demonstrates the superiority of the latter method in small scale conversion with regard to yield and selectivity.
Tetradecane, 3-methyl- Upstream and downstream
18435-22-8 Upstream product
18435-22-8 Downstream Products