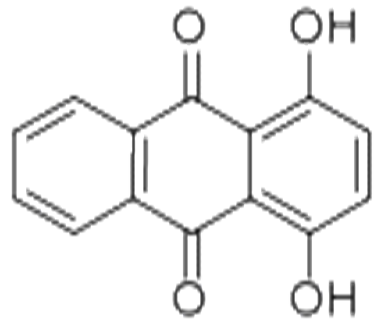
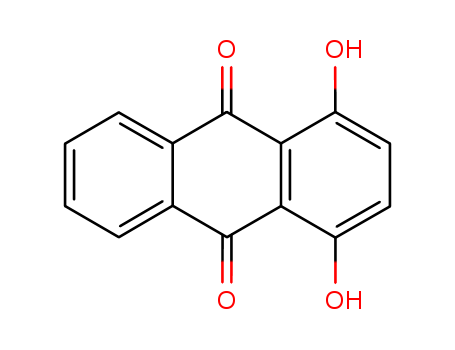
1,4-DIHYDROXYANTHRAQUINONE literature
Clean process for synthesizing 1, 4-dihydroxy anthraquinone
-
Paragraph 0020-0023, (2021/07/08)
The invention relates to the technical field of dye intermediates, and especially relates to a clean process for synthesizing 1, 4-dihydroxy anthraquinone. The process comprises the following steps: sequentially adding 98% sulfuric acid, boric anhydride, phthalic anhydride and hydroquinone into a dry reaction container according to a stoichiometric ratio, uniformly stirring, heating to 100-180 DEG C, and carrying out heat preservation reaction for 2-24 hours; after the heat preservation reaction is finished, cooling the materials, and transferring the materials into another reaction container for hydrolysis; and after hydrolysis is completed, cooling, filtering and washing to obtain the 1, 4-dihydroxy anthraquinone. According to the clean process for synthesizing the 1, 4-dihydroxy anthraquinone, provided by the invention, the dosage of the raw material phthalic anhydride can be reduced, the wastewater treatment difficulty is greatly reduced, and the production cost is also reduced; and meanwhile, the yield of the 1, 4-dihydroxy anthraquinone is also improved to a certain extent.
Synthesis method of orange intermediate
-
Paragraph 0029-0039, (2020/10/21)
The invention discloses a synthesis method of an orange intermediate, and belongs to the field of orange intermediates. The synthesis method of the orange intermediate comprises the following steps: 1, preparing 106-110% sulfuric acid as a solvent; 2, adding p-chlorophenol and excessive phthalic anhydride into a clean reaction container, adding a catalyst boric anhydride, and pouring the sulfuricacid solvent obtained in step 1 into the reaction container; 3, heating the reaction container to 190-200 DEG C, and reacting the p-chlorophenol and phthalic anhydride added in step 2 under the actionof a catalyst; and 4, after the reaction is finished, cooling, diluting, separating out upper wastewater, and filtering to obtain the orange intermediate. According to the scheme, 106-110% sulfuric acid is adopted as the solvent, boric anhydride is adopted as the catalyst, and hydrolysis of phthalic anhydride can be greatly reduced, so the use amount of phthalic anhydride is reduced, the yield isgreatly increased, phthalic anhydride is recycled, and COD of three wastes is greatly reduced.
Evaluation of a series of 9,10-anthraquinones as antiplasmodial agents
Osman, Che Puteh,Ismail, Nor Hadiani,Widyawaruyanti, Aty,Imran, Syahrul,Tumewu, Lidya,Choo, Chee Yan,Ideris, Sharinah
, p. 353 - 363 (2019/06/20)
Background: A phytochemical study on medicinal plants used for the treatment of fever and malaria in Africa yielded metabolites with potential antiplasmodial activity, many of which are Anthraquinones (AQ). AQs have similar sub-structure as naphthoquinones and xanthones, which were previously reported as novel antiplasmodial agents. Objective: The present study aimed to investigate the structural requirements of 9,10-anthraquinones with hydroxy, methoxy and methyl substituents to exert strong antiplasmodial activity and to investigate their possible mode of action. Methods: Thirty-one AQs were synthesized through Friedel-Crafts reaction and assayed for antiplasmodial activity in vitro against Plasmodium falciparum (3D7). The selected compounds were tested for toxicity and probed for their mode of action against β-hematin dimerization through HRP2 and lipid catalyses. The most active compounds were subjected to a docking study using AutoDock 4.2. Results: The active AQs have similar common structural characteristics. However, it is difficult to establish a structure-activity relationship as certain compounds are active despite the absence of the structural features exhibited by other active AQs. They have either ortho- or meta-arranged substituents and one free hydroxyl and/or carbonyl groups. When C-6 is substituted with a methyl group, the activity of AQs generally increased. 1,3-DihydroxyAQ (15) showed good antiplasmodial activity with an IC50 value of 1.08 μM, and when C-6 was substituted with a methyl group, 1,3-dihydroxy-6-methylAQ (24) showed stronger antiplasmodial activity with an IC50 value of 0.02μM, with better selectivity index. Compounds 15 and 24 showed strong HRP2 activity and mild toxicity against hepatocyte cells. Molecular docking studies showed that the hydroxyl groups at the ortho (23) and meta (24) positions are able to form hydrogen bonds with heme, of 3.49 A and 3.02 A, respectively. Conclusion: The activity of 1,3-dihydroxy-6-methylAQ (24) could be due to their inhibition against the free heme dimerization by inhibiting the HRP2 protein. It was further observed that the anthraquinone moiety of compound 24 bind in parallel to the heme ring through hydrophobic interactions, thus preventing crystallization of heme into hemozoin.
Fluorescent molecule for recognizing copper ions, preparation method and application
-
Paragraph 0078; 0079; 0080; 0081, (2019/02/04)
The invention discloses a fluorescent molecule for recognizing copper ions, a preparation method and application. Polycyclic aromatic hydrocarbons such as naphthalene rings or anthracene rings are used as initial raw materials; through a series of optimized organic synthesis reaction (substitution and addition), after the connection with different recognition sites, molecular clamp body tweezer host compounds with different recognition performance can be obtained. The fluorescent molecule can be used for copper ion detection and solves the problems that the existing molecule device is difficult to effectively recognize object molecules.