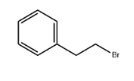
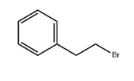
(2-Bromoethyl)benzene literature
Unusually Long Lifetimes of the Singlet Derived from 4-Azido-2,3,5,6-tetrafluorobenzamides
Marcinek, Andrzej,Platz, Matthew S.,Chan, Stephen Y.,Floresca, Rey,Rajagopalan, Krishnan,et al.
, p. 412 - 419 (1994)
Laser flash photolysis (308 nm, 20 ns, 150 mJ) of 4-azido-2,3,5,6-tetrafluorobenzamides, esters, and thioesters generate singlet nitrenes which can be intercepted with pyridine to produce strongly absorbing ylides.It was possible to resolve the rate of formation of the ylides as a function of pyridine concentration.This has lad to direct measurements of the absolute rate constants of (a) the reaction of the nitrene with pyridine, (b) ring expansion of the nitrene to a ketenimine, and (c) singlet to triplet nitrene intersystem crossing.
The oxidation of alcohols to aldehydes and ketones with N-bromosuccinimide in polyethylene glycol: An experimental and theoretical study
Fan, Ji-Cai,Shang, Zhi-Cai,Liang, Jun,Liu, Xiu-Hong,Liu, Yang
, p. 945 - 953 (2008)
Oxidation of aliphatic and aromatic alcohols has been studied by using N-bromosuccinimide (NBS) as oxidant and polyethylene glycol (PEG) as reaction medium under mild reaction conditions. Temperature and solvent effect are studied. Theoretical study is also done with the Gaussian 98 suite of program and the calculation results are in good accordance with the experimental outcome. This system offers a very clean, convenient, environmentally benign method for the oxidation of alcohols. Copyright
-
Walling,Kharasch,Mayo
, p. 2693,2964 (1939)
-
Reactivity of chlorinating agents/PPh3 for the chlorination of alcohols and carboxylic acids: a comparative study
Pluempanupat, Wanchai,Chantarasriwong, Oraphin,Taboonpong, Piyada,Jang, Doo Ok,Chavasiri, Warinthorn
, p. 223 - 226 (2007)
The reactivity of chlorinating agents was examined with the aid of 1H NMR using competitive reactions between selected chlorinating agents and CBr4 towards alcohols and carboxylic acids. The reactivity was greatly dependent on the type of substituent on the chlorinating agents. COCCl3 and CN substituted trichloromethyl groups enhanced the reactivity of the chlorinating agent with PPh3 for the chlorination of alcohols and carboxylic acids.
Selective carboxylation of reactive benzylic C–H bonds by a hypervalent iodine(III)/inorganic bromide oxidation system
Dohi, Toshifumi,Iwasaki, Kosuke,Kita, Yasuyuki,Morimoto, Koji,Tsunoda, Yusuke,Ueda, Shohei
, p. 1087 - 1094 (2018)
An oxidation system comprising phenyliodine(III) diacetate (PIDA) and iodosobenzene with inorganic bromide, i.e., sodium bromide, in an organic solvent led to the direct introduction of carboxylic acids into benzylic C–H bonds under mild conditions. The unique radical species, generated by the homolytic cleavage of the labile I(III)–Br bond of the in situ-formed bromo-λ3-iodane, initiated benzylic carboxylation with a high degree of selectivity for the secondary benzylic position.
-
Hojo,K.,Mukaiyama,T.
, p. 619 - 622 (1976)
-
-
Kharasch,Weinhouse
, p. 209,227 (1936)
-
Evolution of a 4-Benzyloxy-benzylamino Chemotype to Provide Efficacious, Potent, and Isoform Selective PPARα Agonists as Leads for Retinal Disorders
Dou, Xiaozheng,Nath, Dinesh,Shin, Henry,Nurmemmedov, Elmar,Bourne, Philip C.,Ma, Jian-Xing,Duerfeldt, Adam S.
, p. 2854 - 2876 (2020)
Peroxisome proliferator-activated receptor alpha (PPARα) is expressed in retinal Müller cells, endothelial cells, and in retinal pigment epithelium; agonism of PPARα with genetic or pharmacological tools ameliorates inflammation, vascular leakage, neurodegeneration, and neovascularization associated with retinal diseases in animal models. As such, PPARα is a promising drug target for diabetic retinopathy and age-related macular degeneration. Herein, we report proof-of-concept in vivo efficacy in an streptozotocin-induced vascular leakage model (rat) and preliminary pharmacokinetic assessment of a first-generation lead 4a (A91). Additionally, we present the design, synthesis, and evaluation of second-generation analogues, which led to the discovery of 4u and related compounds that reach cellular potencies <50 nM and exhibit >2,700-fold selectivity for PPARα over other PPAR isoforms. These studies identify a pipeline of candidates positioned for detailed PK/PD and pre-clinical evaluation.
Approach to Comparing the Functional Group Tolerance of Reactions
Gensch, Tobias,Teders, Michael,Glorius, Frank
, p. 9154 - 9159 (2017)
Herein, we describe an approach to quantifying and comparing functional group (FG) tolerance of synthetic reactions. Additive-based reaction screening is utilized as a tool for the objective comparison of reaction conditions as demonstrated in four case studies. This contributes to an understanding of factors limiting a reaction's FG tolerance and the identification of truly mild reactions.
Photolytic generation of carbon radicals from Barton esters: Recent developments
Barton,Jaszberenyi,Tang
, p. 3381 - 3384 (1993)
O-Acyl derivatives of N-hydroxy-2-thiopyridone can be photolysed with suitable commercial visible-light lamps to give reactions with very short half-lives (down to 20 s) at 0° C or room temperature.
-
Wiley et al.
, p. 964 (1964)
-
REACTIONS OF BrCl WITH ALKYL RADICALS.
Skell, P. S.,Baxter, H. N.,Tanko, J. M.
, p. 5181 - 5184 (1986)
It is demonstrated that photohalogenation of low reactivity substrates with BrCl occurs mainly with Cl. selectivity.With tertiary or benzylic hydrogens in the substrate, mainly Br. selectivity is observed.These observations are rationalized, taking into account the relative concentrations of halogen atoms and their respective rates of hydrogen abstractions.The resultant radicals react with BrCl to make (RBr/RCl) in ratios between 1 and 15.
Scalable anti-Markovnikov hydrobromination of aliphatic and aromatic olefins
Galli, Marzia,Fletcher, Catherine J.,Del Pozo, Marc,Goldup, Stephen M.
, p. 5622 - 5626 (2016)
To improve access to a key synthetic intermediate we targeted a direct hydrobromination-Negishi route. Unsurprisingly, the anti-Markovnikov addition of HBr to estragole in the presence of AIBN proved successful. However, even in the absence of an added initiator, anti-Markovnikov addition was observed. Re-examination of early reports revealed that selective Markovnikov addition, often simply termed "normal" addition, is not always observed with HBr unless air is excluded, leading to the rediscovery of a reproducible and scalable initiator-free protocol.
α-Bromo Carbonyl Compounds as Promoters for the Synthesis of (2-Bromoethyl)benzene by the Anti-Markovnikov Addition of Hydrogen Bromide to Styrene
Neumann, Ronny,Vega, Fernando de la,Bar-On, Adva
, p. 1315 - 1318 (1995)
The synthesis of (2-bromoethyl)benzene by the anti-Markovnikov addition of gaseous hydrogen bromide to styrene has been found to be promoted by α-bromo carbonyl compounds such as 2-bromo-2-methylpropanal.These compounds were found to catalyze the "abnormal" addition in a variety of solvents such as ethyl acetate, heptane, toluene and dioxane.High concentrations of 2-bromo-2-methylpropanal and hydrogen bromide and low concentrations of styrene favor formation of (2-bromoethyl)benzene.Using the free-radical catalyzed cyclization of 6-bromo-1-hexene as a probe we have found that the 2-bromo-2-methylpropanal does not in itself initiate a free-radical chain reaction by thermal formation of radicals.Instead, radicals may react with 2-bromo-2-methylpropanal to form relatively stable 2-methylpropanal radicals.The presence of such radicals increases the effective length or inhibits termination of the free-radical chain reaction (propagation) and in the case of hydrobromination of styrene raises the yield of (2-bromoethyl)benzene.
-
Horne,Shriner
, p. 4652 (1933)
-
-
Hayami et al.
, p. 1628,1629 (1971)
-
Regio- and stereoselective synthesis of bromoalkenes by homolytic hydrobromination of alkynes with hydrogen bromide
Kumaki, Wataru,Kinoshita, Hidenori,Miura, Katsukiyo
supporting information, (2022/03/07)
Homolytic hydrobromination of terminal and internal alkynes with a commercially available solution of hydrogen bromide in acetic acid has been investigated for regio- and stereoselective synthesis of bromoalkenes. Under an aerobic atmosphere at room temperature, the reaction of ethynylarenes with a small excess of HBr efficiently gave (2-bromoethenyl)arenes with good to high E-selectivity. (Alk-1-ynyl)arenes, or internal alkynes bearing both phenyl and alkyl groups at the sp-carbons also underwent the air-initiated hydrobromination to exhibit high Z-selectivity under kinetic conditions using a half equivalent of HBr.
A General Approach to Deboronative Radical Chain Reactions with Pinacol Alkylboronic Esters
André-Joyaux, Emy,Kuzovlev, Andrey,Renaud, Philippe,Tappin, Nicholas D. C.
, p. 13859 - 13864 (2020/06/10)
The generation of carbon-centered radicals from air-sensitive organoboron compounds through nucleohomolytic substitution at boron is a general method to generate non-functionalized and functionalized radicals. Due to their reduced Lewis acidity, alkylboronic pinacol esters are not suitable substrates. We report their in situ conversion into alkylboronic catechol esters by boron-transesterification with a substoichiometric amount of catechol methyl borate combined with an array of radical chain processes. This simple one-pot radical-chain deboronative method enables the conversion of pinacol boronic esters into iodides, bromides, chlorides, and thioethers. The process is also suitable the formation of nitriles and allylated compounds through C?C bond formation using sulfonyl radical traps. The power of combining radical and classical boron chemistry is illustrated with a modular 5-membered ring formation using a combination of three-component coupling and protodeboronative cyclization.
1,3-Diphenyldisiloxane Enables Additive-Free Redox Recycling Reactions and Catalysis with Triphenylphosphine
Buonomo, Joseph A.,Cole, Malcolm S.,Eiden, Carter G.,Aldrich, Courtney C.
, p. 3583 - 3594 (2020/09/15)
The recently reported chemoselective reduction of phosphine oxides with 1,3-diphenyldisiloxane (DPDS) has opened up the possibility of additive-free phosphine oxide reductions in catalytic systems. Herein we disclose the use of this new reducing agent as an enabler of phosphorus redox recycling in Wittig, Staudinger, and alcohol substitution reactions. DPDS was successfully utilized in ambient-temperature additive-free redox recycling variants of the Wittig olefination, Appel halogenation, and Staudinger reduction. Triphenylphosphine-promoted catalytic recycling reactions were also facilitated by DPDS. Additive-free triphenylphosphine-promoted catalytic Staudinger reductions could even be performed at ambient temperature due to the rapid nature of phosphinimine reduction, for which we characterized kinetic and thermodynamic parameters. These results demonstrate the utility of DPDS as an excellent reducing agent for the development of phosphorus redox recycling reactions.