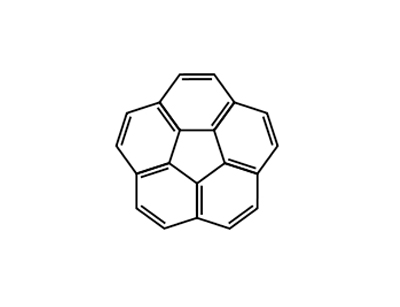
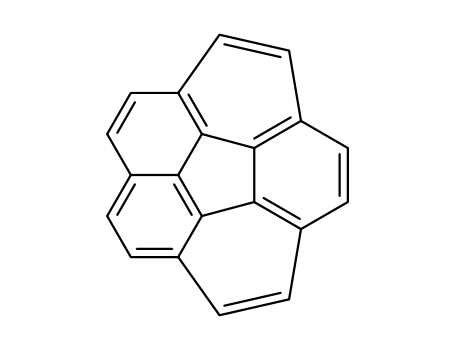
Corannulene literature
Deca-heterosubstituted corannulenes
Pogoreltsev, Alla,Solel, Ephrath,Pappo, Doron,Keinan, Ehud
, p. 5425 - 5427 (2012)
The Cu(i)-catalyzed Ullmann condensation reaction between aliphatic alcohols and sym-pentachlorocorannulene provides a convenient entry to 1,3,5,7,9-pentaalkoxycorannulenes. The latter are easily converted to novel deca-heterosubstituted derivatives, such as 1,3,5,7,9-penta-X-2,4,6,8,10-penta- Y-corannulenes by electrophilic aromatic substitution.
'Buckybowls' - Introducing curvature by solution phase synthesis
Sygula, Andrzej,Xu, Guopin,Marcinow, Zbigniew,Rabideau, Peter W
, p. 3637 - 3644 (2001)
New, non-pyrolytic methods are presented for the synthesis of curved-surface, polynuclear aromatic hydrocarbons related to the fullerenes. Tetrabromocorannulene is conveniently prepared on a large scale and then converted to corannulene, and also used as a synthon for further elaboration.
Corannulene synthesis via the pyrolysis of silyl vinyl ethers
Liu, Charlie Z.,Rabideau, Peter W.
, p. 3437 - 3440 (1996)
The bis(trimethylsilyl enol ether) derived from 7,10-diacetylfluoranthene undergoes pyrolysis to afford corannulene. This is important in cases where the usual precursor, the bis(chlorovinyl) derivative, is not accessible from the diketone.
Chemistry on the rim of buckybowls: Derivatization of 1,2,5,6-tetrabromocorannulene
Xu,Sygula,Marcinow,Rabideau
, p. 9931 - 9934 (2000)
Synthesis of a number of substituted corannulenes and dibenzo[a,g]corannulenes starting from 1,2,5,6-tetrabromocorannulene 3 is described. Since 3 is conveniently obtained by a large scale, non-pyrolytic route, it may serve as a synthon for the further elaboration of this bowl-shaped system. (C) 2000 Elsevier Science Ltd.
Transition metal complexes in organic synthesis, part 55. Synthesis of corannulene via an iron-mediated [2+2+1] cycloaddition
Knoelker, Hans-Joachim,Braier, Arnold,Broecher, Dirk J.,Jones, Peter G.,Piotrowski, Holger
, p. 8075 - 8078 (1999)
The synthesis of corannulene 2 in seven steps and 25% overall yield from 1,8-diiodonaphthalene 3 is reported.
Corannulene. A Convenient New Synthesis
Scott, Lawrence T.,Hashemi, Mohammed M.,Meyer, Dayton T.,Warren, Hope B.
, p. 7082 - 7084 (1991)
-
Reversible phase transitions in a buckybowl monolayer
Merz, Leo,Parschau, Manfred,Zoppi, Laura,Baldridge, Kim K.,Siegel, Jay S.,Ernst, Karl-Heinz
, p. 1966 - 1969 (2009)
Like penguins on ice, buckybowl molecules move closer together when cooled on a copper surface (see model of a corannulene molecule adsorbed on Cu(111)). Upon heating, the molecules spread out into the original crystal phase again. The lower density at ro
A practical, large scale synthesis of the corannulene system [19]
Sygula, Andrzej,Rabideau, Peter W.
, p. 6323 - 6324 (2000)
-
Non-pyrolytic syntheses of buckybowls: Corannulene, cyclopentacorannulene, and a semibuckminsterfullerene
Sygula, Andrzej,Rabideau, Peter W.
, p. 7800 - 7803 (1999)
Corannulene (1), cyclopentacorannulene (2), and a C30H12 semibuckminsterfullerene (3) have been prepared by non-pyrolytic methods employing bromomethyl/dibromomethyl and/or dibromomethyl/dibromomethyl coupling with low-valent titanium or vanadium. Reductive coupling of tetrakis(dibromomethyl)-fluoranthene (8) with vanadium(III) chloride and lithium aluminum hydride affords corannulene in 70-75% yield. Similarly, hexakis(dibromomethyl)fluoranthene (13) leads to cyclopentacorannulene in 20-30% yield, and dodecabromo(octamethyl)indenofluoranthene (6) affords semibuckminsterfullerene (3) in 20% yield.
Emergent Self-Assembly of a Multicomponent Capsule via Iodine Capture
Yang, Yu-Dong,Chen, Xu-Lang,Sessler, Jonathan L.,Gong, Han-Yuan
supporting information, p. 2315 - 2324 (2021/01/13)
Described here is a three-component self-assembly system that displays emergent behavior that differs from that of its constituents. The system comprises an all-hydrocarbon octaaryl macrocycle cyclo[8](1,3-(4,6-dimethyl)benzene (D4d-CDMB-8), corannulene (Cora), and I2. No appreciable interaction is seen between any pair of these three-components, either in cyclohexane or under various crystallization conditions. On the other hand, when all three-components are mixed in cyclohexane and allowed to undergo crystallization, a supramolecular iodine-containing capsule, ((D4d-CDMB-8)3(Cora)2)I2, is obtained. This all-hydrocarbon capsule consists of three D4d-CDMB-8 and two Cora subunits and contains a centrally bound I2 molecule as inferred from single-crystal and powder X-ray diffraction studies as well as solid-state 13C NMR and Raman spectroscopy. These analyses were complemented by solution-phase 1H NMR and UV-vis spectroscopic studies. No evidence of I2 escape from the capsule is seen, even at high temperatures (e.g., up to 418 K). The bound I2 is likewise protected from reaction with alkali or standard reductants in aqueous solution (e.g., saturated NaOH(aq) or aqueous Na2S2O3). It was also found that a mixed powder containing D4d-CDMB-8 and Cora in a 3:2 molar ratio could capture saturated I2 vapor or iodine from aqueous sources (e.g., 1.0 mM I2 in NaCl (35 wt %) or I2 + NaI(aq) (1.0 mM each)). The present system displays structural and functional features that go beyond what would be expected on the basis of a simple sum-of-the-components analysis. As such, it illustrates a new approach to creating self-assembled ensembles with emergent features.
Stack the Bowls: Tailoring the Electronic Structure of Corannulene-Integrated Crystalline Materials
Rice, Allison M.,Dolgopolova, Ekaterina A.,Yarbrough, Brandon J.,Leith, Gabrielle A.,Martin, Corey R.,Stephenson, Kenneth S.,Heugh, Rebecca A.,Brandt, Amy J.,Chen, Donna A.,Karakalos, Stavros G.,Smith, Mark D.,Hatzell, Kelsey B.,Pellechia, Perry J.,Garashchuk, Sophya,Shustova, Natalia B.
supporting information, p. 11310 - 11315 (2018/08/11)
We report the first examples of purely organic donor–acceptor materials with integrated π-bowls (πBs) that combine not only crystallinity and high surface areas but also exhibit tunable electronic properties, resulting in a four-orders-of-magnitude conductivity enhancement in comparison with the parent framework. In addition to the first report of alkyne–azide cycloaddition utilized for corannulene immobilization in the solid state, we also probed the charge transfer rate within the Marcus theory as a function of mutual πB orientation for the first time, as well as shed light on the density of states near the Fermi edge. These studies could foreshadow new avenues for πB utilization for the development of optoelectronic devices or a route for highly efficient porous electrodes.
Electrochemically active porous organic polymers based on corannulene
Karunathilake, Arosha A.K.,Thompson, Christina M.,Perananthan, Sahila,Ferraris, John P.,Smaldone, Ronald A.
supporting information, p. 12881 - 12884 (2016/11/06)
For the first time, porous organic polymers (POPs) based on the smallest buckybowl, corannulene (BB-POPs) have been synthesized. Three POPs were synthesised via Sonogashira co-polymerization of 1,2,5,6-tetrabromocorannulene and alkyne linkers. BB-POP-3 exhibits the highest surface area (SABET = 560 m2 g-1) and CO2 adsorption of 11.7 wt%, while they retain the redox properties of corannulene.
Superaromatic terpyridines based on corannulene responsive to metal ions
Wu, Difeng,Shao, Tao,Men, Jian,Chen, Xiaochuan,Gao, Guowei
supporting information, p. 1753 - 1761 (2014/01/06)
Two superaromatic terpyridine ligands (1 and 2) incorporating a corannulene unit at the 4′-position are reported. The optical and metal sensing properties of both ligands were investigated by the naked eye, and UV-vis and fluorescence spectroscopy in this work. In 1, the corannulene motif is directly connected to the 4′-phenylterpyridine domain, while in 2, the corannulene motif and the 4′-phenylterpyridine domain are separated by an acetylene linker. Both 1 and 2 can work as chemosensors for metal ions and display different optical responses to various metal ions. It is shown that both ligands exhibit a colorimetric sensing ability for Fe2+ through an obvious color change from colorless to magenta, and this color change can be observed easily by the naked eye. The addition of Fe2+ also leads to significant changes in the absorption spectra of the ligands. A characteristic red shift in the emission spectra is observed in the presence of Zn 2+, which facilitates the discrimination of Zn2+ from other metal ions. In addition, density functional theory (DFT) and time-dependent-density functional theory (TD-DFT) calculations were performed and shown to be consistent with the observed experimental results. The Royal Society of Chemistry.