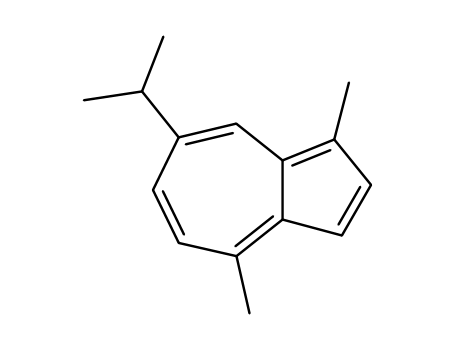
Guaiazulene literature
ALISMOL AND ALISMOXIDE, SESQUITERPENOIDS OF ALISMA RHIZOMES
Oshima, Yoshiteru,Iwakawa, Tsuneo,Hikino, Hiroshi
, p. 183 - 186 (1983)
From the crude drug 'takusha', wich is the rhizomes of Alisma plantago-aquatica var. orientale, two new sesquiterpenoids, alismol and alismoxide, have been isolated.Their structures have been estabished on the basis of chemical and physical evidence to be 1β,5β-guaia-6,10(15)-dien-4-ol and 4,10-epoxy-1β,5β-guaia-6-ene, respectively. - Key Word Index: Alisma plantago-aquatica var. orientale; Alismataceae; sesquiterpenoid; guaiane skeleton; alismol; 1β,5β-guaia-6,10(15)-dien-4-ol; alismoxide; 4,10-epoxy-1β,5β-guaia-6-ene
-
Ukita et al.
, p. 4584,4586 (1954)
-
Reaction of azulene with 1,2-bis[4-(dimethylamino)phenyl]-1,2-ethanediol in a mixed solvent of methanol and acetonitrile in the presence of hydrochloric acid: A facile one-pot synthesis and properties of new triarylethylenes possessing an azulen-1-yl group
Takekuma, Shin-Ichi,Kaibara, Masamichi,Minematsu, Toshie,Takekuma, Hideko
experimental part, p. 4780 - 4792 (2011/08/03)
Reaction of azulene (1) with 1,2-bis[4-(dimethylamino)phenyl]-1,2- ethanediol (2) in a mixed solvent of methanol and acetonitrile in the presence of 36% hydrochloric acid at 60 °C for 3 h gives 2-(azulen-1-yl)-1,1-bis[4- (dimethylamino)phenyl]ethylene (3) (8% yield), 1-(azulen-1-yl)-(E)-1,2-bis[4- (dimethylamino)phenyl]ethylene (4) (28% yield), and 1,3-bis{2,2-bis[4- (dimethylamino)phenyl]ethenyl}azulene (5) (9% yield). Besides the above products, this reaction affords 1,1-di(azulen-1-yl)-2,2-bis[4-(dimethylamino) phenyl]ethane (6) (15% yield), a meso form (1R,2S)-1,2-di(azulen-1-yl)-1,2- bis[4-(dimethylamino)phenyl]ethane (7) (6% yield), and the two enantiomeric forms (1R,2R)- and (1S,2S)-1,2-di(azulen-1-yl)-1,2-bis[4-(dimethylamino)phenyl] ethanes (8) (6% yield). Furthermore, addition reaction of 3 with 1 under the same reaction conditions as the above provides 6, in 46% yield, which upon oxidation with DDQ (=2,3-dichloro-5,6-dicyano-1,4-benzoquinone) in dichloromethane at 25 °C for 24 h yields 1,1-di(azulen-1-yl)-2,2-bis[4- (dimethylamino)phenyl]ethylene (9) in 48% yield. Interestingly, reaction of 1,1-bis[4-(dimethylamino)phenyl]-2-(3-guaiazulenyl)ethylene (11) with 1 in a mixed solvent of methanol and acetonitrile in the presence of 36% hydrochloric acid at 60 °C for 3 h gives guaiazulene (10) and 3, owing to the replacement of a guaiazulen-3-yl group by an azulen-1-yl group, in 91 and 46% yields together with 5 (19% yield) and 6 (13% yield). Similarly, reactions of 2-(3-guaiazulenyl)-1,1-bis(4-methoxyphenyl)ethylene (12) and 1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}-2-(3-guaiazulenyl)ethylene (13) with 1 under the same reaction conditions as the above provide 10, 2-(azulen-1-yl)-1,1-bis(4-methoxyphenyl)ethylene (16), and 1,3-bis[2,2-bis(4- methoxyphenyl)ethenyl]azulene (17) (93, 34, and 19% yields) from 12 and 10 and 2-(azulen-1-yl)-1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}ethylene (18) (97 and 58% yields) from 13.
Approach to the blues: A highly flexible route to the azulenes
Carret, Sebastien,Blanc, Aurelien,Coquerel, Yoann,Berthod, Mikael,Greene, Andrew E.,Depres, Jean-Pierre
, p. 5130 - 5133 (2007/10/03)
(Chemical Equation Presented) A palette of blues: Chlorobicyclo-[5.3.0] decatrienones are readily prepared from cycloheptatrienes by cycloaddition of dichloroketene, ring expansion with a diazoalkane, and dehydrochlorination in dimethylformamide. These compounds are used as intermediates in the regiocontrolled synthesis of a wide variety of natural and nonnatural azulenes (see scheme).
Chemistry of (+)-aromadendrene. Part 6: Rearrangement reactions of ledene, isoledene and their epoxides
Moreno-Dorado, F. Javier,Lamers, Yvonne M. A. W.,Mironov, Grigore,Wijnberg, Joannes B. P. A.,De Groot, Aede
, p. 7743 - 7750 (2007/10/03)
The chemistry of (+)-ledene and (-)-isoledene, both easily available from (+)-aromadendrene has been investigated. Reactions at the double bond of ledene take place preferably from the β-side. Under acidic conditions its C7-C8 β-epoxide and β-diol preferably react via carbocations, which are initially formed at C8. Rearrangement takes place to compounds with cubebane and cadinane skeletons. The reaction pattern of isoledene and its α-epoxide, under acidic conditions, is governed by the easy formation of an intermediate α-cyclopropylcarbinyl carbocation. Further reactions lead to products in which the C2-C3 bond of the cyclopropane ring is broken to give compounds with a guaiane skeleton. Guaiane-type dienes and unsaturated cyclic ethers are the final products of these rearrangements. Several derivatives of these compounds have been prepared.
Formation and Thermal Rearrangement of Dimethyl Tricyclo<6.2.2.01,7>dodeca-2,4,6,9,11-pentaene-9,10-dicarboxylates
Fallahpour, Reza-Ali,Hansen, Hans-Juergen
, p. 1933 - 1970 (2007/10/02)
The high-pressure reaction of 1-methylazulenes 1 with excess of dimethyl acetylenedicarboxylate (ADM) in hexane at 30 deg and at pressures up to 7 kbar affords the tricyclic compounds 2 in reasonable-to-good yields (cf.Table 1).The crystalline compounds 2 decompose on melting into the starting materials and undergo rearrangement to the corresponding heptalene-1,2-dicarboxylates 6.The X-ray crystal-structure analyses of 2f and 2g (cf.Fig. 1) reveal the presence of a perfectly planar seven-membered ring and comparably long C(1)-C(10) as well as C(1)-C(11) bonds (cf.Tables 2 and 3).The thermolysis of 2g in different solvents leads in aprotic media to the formation of the starting azulene 1g and, depending upon the polarity of the solvents, to varying amounts of the corresponding heptalene-1,2-dicarboxylate 6f (cf.Table 11).The formed amounts of 1g depends linearly on the ET values of the solvents (cf.Fig. 4).The same is valid for the thermolysis of 2g in protic media (cf.Table 10 and Fig. 3).However, in these cases instead of the heptalene-1,2-dicarboxylate 6g, the corresponding (E)- and (Z)-isomers of the 1-(azulen-1-yl)ethene-1,2-dicarboxylates 7g are formed.The other tricyclic compounds 2 exhibit the same behavior on thermolysis in MeCN and BuOH (cf.Tables 8 and 9, resp.).The results show that the tricyclic compounds 2 undergo at temperatures up to 110 deg C two competing reactions, namely heterolysis of the C(1)-C(10) bond, leading to the formation of heptalenes 6 in polar aprotic media, and (E)- and (Z)-ethene-1,2-dicarboxylates 7 in polar protic media, and concerted homolysis of the C(1)-C(10) and C(8)-C(9) bonds in the sense of a retro-Diels-Alder reaction in apolar media, yielding the starting azulenes and ADM, the amount of which decrease with increasing polarity of the solvent.The kinetic and activation parameters measured for 2g and the other tricyclic compounds 2 are collected in Tables 12-15.The tricyclic compounds 2a and 2b show in polar aprotic media (MeCN) a different behavior in that they form, instead of heptalenes, the corresponding 3,4-dihydrocyclopentazulene-1,2-dicarboxylates 16a and 16b, respectively (Scheme 4).Experiments with <8-2H>-2a showed that these compounds are not formed via intramolecular H-shifts (cf.Schemes 8 and 9).