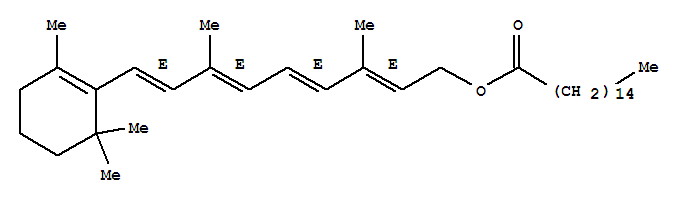
Retinol palmitate literature
Antioxidant activity and synergism of butylhydroxytoluene in processes of oxidation of retinol esters
Finkel'shtein,Mednikova,Alekseev,Kozlov
, p. 100 - 104 (1977)
-
Synthesis of retinyl palmitate catalyzed by Candida sp.99-125 lipase immobilized on fiber-like SBA-15 mesoporous material
Zhu, Kai,Wang, Jianqiang,Wang, Yan-Hua,Liu, Hui,Han, Ping-Fang,Wei, Ping
, p. 7593 - 7602 (2011)
Candida sp.99-125 lipase was suitable for transesterification of fats and oils to produce fatty acid methyl ester. The adsorption of Candida sp.99-125 lipase onto the fiber-like SBA-15 mesoporous material has been studied. The unaltered structural order of the fiber-like SBA-15 before and after the adsorption has been confirmed by FT-IR, SEM and N2 adsorption. The amount of adsorbed Candida sp.99-125 lipase depends both on the solution pH and reaction time. Good adsorption capacity of Candida sp.99-125 lipase on fiber-like SBA-15 may be due to solution pH from 5.0 to 9.0 especially at 7.0 (93.99 mg enzyme per gram silica is obtained and the activity recovery is 281.05%). A high lipase loading (135.9 mg enzyme per gram silica) was obtained, but it did not produce a proportionate level of catalytic activity. The immobilized Candida sp.99-125 lipase showed increased adaptability in the hydrolysis of p-nitrophenyl acetate compared to free Candida sp.99-125 lipase at pH 5.0-9.0. Meanwhile, the immobilized Candida sp.99-125 lipase showed higher thermal stability than that of free Candida sp.99-125 lipase. And the synthesis of retinyl palmitate in organic solvent with the immobilized Candida sp.99-125 lipase was investigated. The influence factors, such as: the solvent used, the molar ratio and concentrations of substrates, the reaction time and the amount of lipase were studied and optimized. In the conditions of transesterificating 0.164 g retinyl acetate and 0.32 g palmitic acid, 10 mL of solvent hexane, 1:4 of mass ratio of lipase to retinyl acetate, and 6 hours of reaction time, 74.6% of retinyl acetate was converted into retinyl plamitate. Copyright
Synthesis method of 4-palmitoyloxy-2-methyl-2-butenal and synthesis method of vitamin A palmitate
-
, (2021/04/10)
The invention discloses a synthesis method of a vitamin A palmitate intermediate 4-palmitoyloxy-2-methyl-2-butenal, application of the vitamin A palmitate intermediate 4-palmitoyloxy-2-methyl-2-butenal in vitamin A palmitate synthesis, and a method for synthesizing vitamin A palmitate by using the intermediate. According to the method, in the process of introducing aldehyde group, solvent-free reaction conditions are adopted, so that the use of organic solvents is avoided while the reaction yield is increased, the production cost is reduced, the operation is simple and convenient, and the method is suitable for industrial large-scale production.
Method for preparing vitamin A and vitamin A ester
-
, (2020/04/17)
The invention provides a novel method for preparing vitamin A and vitamin A ester by taking farnesol as a raw material. The method comprises the following steps: carrying out oxidation reaction on farnesol and oxygen under the action of a catalyst and a cocatalyst to generate farnesal; carrying out dehydrogenation reaction on farnesal to generate dehydrofarnesal; carrying out cyclization reactionon the dehydrofarnesal under the catalysis of acid to generate a cyclized intermediate; carrying out a reaction on the cyclized intermediate with chloroisopentenol to generate vitamin A; carrying outan esterification reaction on vitamin A to generate vitamin A ester. The method avoids the defects of an existing process, and the process line is economical and effective.
Preparation method of vitamin A ester intermediate C15 and vitamin A ester
-
, (2020/08/18)
The invention provides a preparation method of a vitamin A ester intermediate C15 and vitamin A ester. The method comprises the following steps: carrying out a halogenation reaction and a cyclizationreaction on 3, 7-dimethyl-3-hydroxy-1, 6-octadiene as an initial raw material, carrying out a substitution reaction on the obtained product and triphenylphosphine or triester phosphite to prepare a corresponding Wittig reagent, carrying out a Wittig reaction on the Wittig reagent and 2-methyl-4-acetoxy-2-butenal, performing acidifying, hydrolyzing and acidifying the obtained product, and carryingout a substitution reaction on the hydrolyzed and acidified product and triphenylphosphine or triester phosphite to prepare C15. The vitamin A ester can be prepared by carrying out a Wittig reaction on the obtained C15 and 2-methyl-4-R3 substituent carbonyloxy-2-butenal. The method has the advantages of single reaction type, easy operation and realization of reaction conditions, safe and environment-friendly operation, simple post-treatment and low cost; and the reaction activity is strong, the reaction selectivity is high, the atom economy is high, and the target product yield and purity arehigh.
Preparation method of vitamin A palmitate
-
Paragraph 0017-0028, (2018/10/27)
The invention belongs to the technical field of medicines, in particular to preparation of vitamin A palmitate and a composition thereof. Aiming at solving the technical problem, the invention provides a preparation method of vitamin A palmitate. The preparation method comprises the steps of carrying out alcoholysis on vitamin A acetate with an alcohol solvent to obtain retinol, and then adding methyl palmitate for alcoholysis again to obtain vitamin A palmitate. The preparation method is simple in process, is environmentally friendly, and has high purity and high yield.