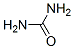Urea literature
Real-Time in Vivo Detection of H2O2 Using Hyperpolarized 13C-Thiourea
Wibowo, Arif,Park, Jae Mo,Liu, Shie-Chau,Khosla, Chaitan,Spielman, Daniel M.
, p. 1737 - 1742 (2017)
Reactive oxygen species (ROS) are essential cellular metabolites widely implicated in many diseases including cancer, inflammation, and cardiovascular and neurodegenerative disorders. Yet, ROS signaling remains poorly understood, and their measurements are a challenge due to high reactivity and instability. Here, we report the development of 13C-thiourea as a probe to detect and measure H2O2 dynamics with high sensitivity and spatiotemporal resolution using hyperpolarized 13C magnetic resonance spectroscopic imaging. In particular, we show 13C-thiourea to be highly polarizable and to possess a long spin-lattice relaxation time (T1), which enables real-time monitoring of ROS-mediated transformation. We also demonstrate that 13C-thiourea reacts readily with H2O2 to give chemically distinguishable products in vitro and validate their detection in vivo in a mouse liver. This study suggests that 13C-thiourea is a promising agent for noninvasive detection of H2O2 in vivo. More broadly, our findings outline a viable clinical application for H2O2 detection in patients with a range of diseases.
-
Walker,Kay
, p. 489 (1897)
-
-
Walker,Wood
, p. 33 (1900)
-
Oxidation of Thiourea by Aqueous Bromine: Autocatalysis by Bromide
Simoyi, Reuben H.,Epstein, Irving R.
, p. 5124 - 5128 (1987)
The reaction between thiourea and aqueous bromine was studied in the pH range 1.5-4.The reaction occurs in two stages: a very fast initial stage in which 1 mol of bromine is consumed for each mole of thiourea, followed by a slower second stage in which the rest of the bromine is consumed.The stoichiometry of the reaction at pH >/= 2 is 4Br2 + CS(NH2)2 + 5H2O -> 8Br- + CO(NH2)2 + SO42- + 10 H+.At pH 8Br- + 2NH4+ + SO42- + CO2 + 8H+.The second stage of the reaction is autocatalytic in bromide.At 25.0 +/- 0.1 deg C and ionic strength 0.2 M (NaClO4), the rate expression is -1/3d/dt = (k1 + k2->), with k1 = 27.8 +/- 0.5 M-1 s-1 and k2 = (3.17 +/- 0.3) x 1E3 M-2 s-1.This reaction is explained by successive electrophilic attacks on the sulfur center by bromine.
-
Surrey,Nachod
, p. 2336 (1951)
-
Fingering Patterns and Other Interesting Dynamics in the Chemical Waves Generated by the Chlorite-Thiourea Reaction
Chinake, Cordelia R.,Simoyi, Reuben H.
, p. 4012 - 4019 (1994)
The reaction between chlorite and thiourea is excitable and autocatalytic in HOCl.It produces a chemical wave of ClO2 when ClO2(-) is in stoichiometric excess over thiourea.The chemical wave has been studied in glass tubes of varying diameters.The dynamics of the front propagation have been studied as a function of convection, which is known to induce density gradients.The ClO2(-)-thiourea reaction is highly exothermic, and the chemical wave has a positive isothermal density change.In vertical tubes the effect of the exothermicity of the reaction opposes the effect of the isothermal density change, giving an asymmetric and unstable wave front in descending waves.Multicomponent convection and fingering patterns heve been observed in descending waves.Ascending waves propagate without structure and are generally slower than descending waves.In strach solutions fingering patterns are observed which propagate downward at greater than 10 times the normal front velocity.These fingers turn into rapidly-rising plumes after they reach the botton of the tube.Formation of rising plumes is due to the hot interior of the finger which is lighter than the unreacted solution, but when the reacted solution propagates upward into the cold unreacted region, the cooling effect makes the solution heavier, giving a symmetric "mushroom-shaped" plume.
Evidence for an inhibitory LIM domain in a rat brain agmatinase-like protein
Castro, Victor,Fuentealba, Pablo,Henriquez, Adolfo,Vallejos, Alejandro,Benitez, Jose,Lobos, Marcela,Diaz, Beatriz,Carvajal, Nelson,Uribe, Elena
, p. 107 - 110 (2011)
We recently cloned a rat brain agmatinase-like protein (ALP) whose amino acid sequence greatly differs from other agmatinases and exhibits a LIM-like domain close to its carboxyl terminus. The protein was immunohistochemically detected in the hypothalamic region and hippocampal astrocytes and neurons. We now show that truncated species, lacking the LIM-type domain, retains the dimeric structure of the wild-type protein but exhibits a 10-fold increased kcat, a 3-fold decreased Km value for agmatine and altered intrinsic tryptophan fluorescent properties. As expected for a LIM protein, zinc was detected only in the wild-type ALP (~2 Zn2+/monomer). Our proposal is that the LIM domain functions as an autoinhibitory entity and that inhibition is reversed by interaction of the domain with some yet undefined brain protein.
-
Hofmeister
, (1896)
-
-
Walker,Hambly
, p. 746 (1895)
-
Aminoguanidinium hydrolysis effected by a hydroxo-bridged Dicobalt(II) complex as a functional model for arginase and catalyzed by mononuclear Cobalt(II) complexes
He, Chuan,Lippard, Stephen J.
, p. 105 - 113 (1998)
The dinuclear complex [Co2(μ-OH)(μ-XDK)(bpy)2(EtOH)](NO3), where XDK is the dinucleating dicarboxylate ligand m-xylylenediamine bis(Kemp's triacid imide) and bpy = 2,2'-bipyridine, was prepared as a functional model for arginase. The substrate aminoguanidinium nitrate was hydrolyzed to urea in ethanol by the complex but not by free hydroxide ion under the same conditions. The amino group of the substrate binds to cobalt, as demonstrated by W-vis spectroscopic studies. The syntheses of related dinuclear cobalt(II) complexes [Co2(μ-XDK)(NO3)2(CH3OH)2(H2O)], [Co2(μ-Cl)(μ-XDK)(bpy)2(EtOH)2](NO3), and [Co2(μ-XDK)-(py)3(NO3)2] are described. Mononuclear complexes [Co(XDK)(bpy)(H2O)] and [Zn(XDK)(bpy)(H2O)] were also prepared and characterized. The former catalytically hydrolyzes aminoguanidinium nitrate to urea in basic 1:1 methanol/water solutions, whereas the latter does not promote this reaction. Hydrolysis of aminoguanidinium ion is effected by [Co(CH3COO)2] and [Cu(CH3COO)2] in the presence of bpy, but not by [Zn(CH3COO))2], [Ni(CH3COO)2], or [Mn(CH3COO)2] in the presence of bpy in 1:1 methanol/water solution. In all cases, coordination of the amino group of the substrate to the metal center under the reaction conditions may activate the leaving group and orient the guanidinium moiety close to the attacking nucleophile, metal-bound hydroxide ion, to promote the hydrolysis reaction.
Photocatalytic synthesis of urea from in situ generated ammonia and carbon dioxide
Srinivas, Basavaraju,Kumari, Valluri Durga,Sadanandam, Gullapelli,Hymavathi, Chilumula,Subrahmanyam, MacHiraju,De, Bhudev Ranjan
, p. 233 - 241 (2012)
TiO2 and Fe-titanate (different wt%) supported on zeolite were prepared by sol-gel and solid-state dispersion methods. The photocatalysts prepared were characterized by X-ray diffraction, scanning electron microscopy and ultraviolet (UV)-visible diffuse reflectance spectroscopy techniques. Photocatalytic reduction of nitrate in water and isopropanol/oxalic acid as hole scavengers are investigated in a batch reactor under UV illumination. The yield of urea increased notably when the catalysts were supported on zeolite. The Fe-titanate supported catalyst promotes the charge separation that contributes to an increase in selective formation of urea. The product formation is because of the high adsorption of in situ generated CO2 and NH3 over shape-selective property of the zeolite in the composite photocatalyst. The maximum yield of urea is found to be 18 ppm while 1% isopropanol containing solution over 10 wt% Fe-titanate/HZSM-5 photocatalyst was used.
-
Svirbely,Peterson
, p. 166 (1943)
-
-
Cumming
, p. 1391 (1903)
-
-
Clark,Gaddy,Rist
, p. 1092 (1933)
-
Oxyhalogen-sulfur chemistry: Kinetics and mechanism of oxidation of formamidine disulfide by acidic bromate
Madhiri, Nicholas,Olojo, Rotimi,Simoyi, Reuben H.
, p. 4149 - 4156 (2003)
The kinetics and mechanism of the oxidation of formamidine disulfide, FDS, a dimer and major metabolite of thiourea, by bromate have been studied in acidic media. In excess bromate conditions the reaction displays an induction period before formation of bromine. The stoichiometry of the reaction is: 7BrO3- + 3[(H2N(HN=)CS-]2 + 9H 2O → 6NH2CONH2 + 6SO4 2- + 7Br- + 12H- (A). In excess oxidant conditions, however, the bromide formed in reaction A reacts with bromate to give bromine and a final stoichiometry of: 14BrO3- + 5[(H2N(HN=)CS-]2 + 8H2O → 10NH 2CONH2 + 10SO42- + 7Br2 + 6H+ (B). The direct reaction of bromine and FDS was also studied and its stoichiometry is: 7Br2 + [(H2N(HN=)CS-] 2 + 10H2O → 2NH2CONH2 + 2SO42- + 14Br- + 18H+ (C). The overall rate of reaction A, as measured by the rate of consumption of FDS, is second order in acid concentrations, indicating the dominance of oxyhalogen kinetics which control the formation of the reactive species HBrO2 and HOBr. The reaction proceeds through an initial cleavage of the S-S bond to give the unstable sulfenic acids which are then rapidly oxidized through the sulfinic and sulfonic acids to give sulfate. The formation of bromine coincides with formation of sulfate because the cleavage of the C-S bond to give sulfate occurs at the sulfonic acid stage only. The mechanism derived is the same as that derived for the bromate-thiourea reaction, suggesting that FDS is an intermediate in the oxidation of thiourea to its oxo-acids as well as to sulfate.
Kinetics and Mechanism of the Complex Oxidation of Aminoiminomethanesulfinic Acid by Iodate in Acidic Medium
Mambo, Elizabeth,Simoyi, Reuben H.
, p. 13662 - 13667 (1993)
The reaction between iodate and aminoiminomethanesulfinic acid, NH2(NH)CSO2H (AIMSA), has been studied in acidic medium.The stoichiometry of the reaction in excess AIMSA is 2IO3- + 3AIMSA + 3H2O -> 3SO42- + 3CO(NH2)2 + 2I- + 6H+ (eq 1), and the soichiometry of the reaction in excess iodate is 4IO3- + 5AIMSA + 3H2O -> 5SO42- + 5CO(NH2)2 + 2I2 + +6H+ (eq 2).In excess AIMSA and high acid concentrations the reaction shows an induction period and a transient formation of iodine, while in excess iodate concentrations iodine is produced and partially consumed, leaving a finite iodine concentration at the end of the reaction.The dynamics of the reaction is explained by a combination of three reactions: the first is the oxidation of AIMSA by iodate to give iodide, the second is the Dushman reaction which forms iodine from the iodate-iodide reaction, and the third is the reaction of iodine and AIMSA.The relative rates of these three reactions will determine the dynamics of the reaction.The oxidation of AIMSA with I2 and I3- was also investigated.The oxidation of AIMSA by I2 and I3- was found to be inhibited by acid because the oxidation of AIMSA by HOI is faster than that with molecular I2.The reaction is also autoinhibitory because the product of the reaction, I-, combines with unreacted I2 to form I3- which is relatively inert toward AIMSA.A computer simulation study is performed to enhance the proposed mechanism.
-
Palm,Calvin
, p. 2115 (1962)
-
The kinetics of pentoxyl oxidation by hypochlorite ions
Kheidorov,Ershov,Zyabkina
, p. 353 - 356 (2006)
The kinetics of pentoxyl (I) oxidation in aqueous media under the action of hypochlorite ions was studied at pH 8.8 and 273-298 K. The order of the reaction with respect to both participants was found to be one. The temperature dependence of the reaction rate obeyed the Arrhenius law. The reaction activation parameters were found to be E a=11.08 kJ/mol, ΔH ≠=8.73 kJ/mol, ΔS ≠=-200.70 J/(mol K), and ΔG ≠=66.88 kJ/mol. Reaction stoichiometry was studied, the chemical characteristics of the process considered, and a mechanism of the oxidative transformation of I under the action of OCl- suggested. Pleiades Publishing, Inc., 2006.
-
Schwander,Cordebard
, (1930)
-
Oxyhalogen-Sulfur Chemistry: The Bromate-(Amininoimino)methanesulfinic Acid Reaction in Acidic Medium
Chinake, Cordelia R.,Simoyi, Reuben H.,Jonnalagadda, Sreekantha B.
, p. 545 - 550 (1994)
The reaction between (amnoimino)methanesulfinic acid, HO2SC(NH)NH2(AIMSA), and bromate has been studied in acidic medium.In excess AIMSA the stoichiometry of the reaction is 2BrO3- + 3AIMSA + 3H2O -> 3SO42- + 3CO(NH2)2 + 2Br- + 6H+, and in excess bromate the stoichiometry is 4BrO3- + 5AIMSA + 3H2O -> 5SO42- + 5CO(NH2)2 + 2Br2 + 6H+.Br2 is produced only when BrO3- is in stoichiometric excess over AIMSA.It is produced from the reaction of the product, Br-, with excess BrO3- after all the AIMSA has been consumed.The reaction has an initial induction period followed by formation of bromine.Although AIMSA is oxidized to SO42-, no SO42- formation is observed until Br2 production commences.The reaction is autocatalyzed by bromide.The reactive oxidizing species in solution are HOBr and Br2.Bromide enhances their formation from bromate.A simple eight-reaction mechanism is used to describe the reaction.The reaction commences through a direct reaction between BrO3- and AIMSA: BrO3+ + HO2SC(NH)NH2 + H+ -> HBrO2 + HO3SC(NH)NH2 with k = 2.5E-2M-2s-1.The rate-determining step is the standard BrO3- - Br- reaction which forms the reactive species HOBr:BrO3- + Br- + 2H+ -> HBrO2 + HOBr.A computer simulation analysis of the proposed mechanism gave good fit to the data.
-
Krase,Gaddy,Clark
, p. 289 (1930)
-
Spectroscopic study of photo and thermal destruction of riboflavin
Astanov, Salikh,Sharipov, Mirzo Z.,Fayzullaev, Askar R.,Kurtaliev, Eldar N.,Nizomov, Negmat
, p. 133 - 138 (2014)
Influence of temperature and light irradiation on the spectroscopic properties of aqueous solutions of riboflavin was studied using linear dichroism method, absorption and fluorescence spectroscopy. It was established that in a wide temperature range 290-423 K there is a decline of absorbance and fluorescence ability, which is explained by thermodestruction of riboflavin. It is shown that the proportion of molecules, which have undergone degradation, are in the range of 4-28%, and depends on the concentration and quantity of temperature effects. Introduction of hydrochloric and sulfuric acids, as well as different metal ions leads to an increase in the photostability of riboflavin solutions by 2-2.5 times. The observed phenomena are explained by the formation protonation form of riboflavin and a complex between the metal ions and oxygen atoms of the carbonyl group of riboflavin, respectively.
-
Williams
, (1868)
-
-
Fichter
, p. 31,96 (1930)
-
Formation of adenine from CH3COONH4/NH 4HCO3-the probable prebiotic route for adenine
Singh, Palwinder,Singh, Amrinder
, p. 2525 - 2527 (2013)
Adenine was formed when an aqueous solution of CH3COONH 4/NH4HCO3 was subjected to mass spectrometer/refluxed for 72 h/heated in a closed vessel for a long time. Since these salts are sources of CO2 and NH3 and H2O is available from the reaction medium, adenine might get formed by the combination of CO2, H2O and NH3. The occurrence of this reaction in the gas phase as well as in the aqueous phase points towards the possibility of similar reactions during the primitive earth conditions.
Decomposition of Thiourea Dioxide under Aerobic and Anaerobic Conditions in an Aqueous Alkaline Solution
Egorova, E. V.,Nikitin, K. S.,Polenov, Yu. V.
, p. 2038 - 2041 (2020)
Abstract: The kinetics and mechanism of the decomposition of thiourea dioxide in an aqueous alkaline solution under aerobic and anaerobic conditions are established. It is discovered that along with the decomposition of thiourea dioxide molecules with C–S bond cleavage and the subsequent formation of sulfoxyl acid anions, there is a reversible stage of the formation of thiourea and peroxide anions. The rate constants of the indicated stages are determined via mathematical modeling using the experimental data.
-
Jaenecke
, (1930)
-
-
Franz,Applegath
, p. 3304 (1961)
-
-
Gal'perin,Finkel'shtein
, (1971)
-
-
Inoue et al.
, p. 1339,1344 (1972)
-
-
Frejacques
, (1948)
-
-
Jaffe
, p. 398 (1890)
-
-
Pfaltz,Baudisch
, p. 2972 - 2979 (1923)
-
Degradation of 2-ketoarginine by guanidinobutyrase in arginine aminotransferase pathway of Brevibacterium helvolum.
Yorifuji,Kaneoke,Okazaki,Shimizu
, p. 512 - 513 (1995)
Guanidinobutyrase (EC 3.5.3.7) involved in the arginine oxygenase pathway of Brevibacterium helvolum IFO 12073 was found to catalyze also the hydrolysis of 2-ketoarginine (2-keto-5-guanidinovalerate) to 2-ketoornithine (2-keto-5-aminovalerate) and urea, the second step of the arginine aminotransferase pathway. No other enzyme that degraded 2-ketoarginine was found in cells grown on L-arginine. The enzyme hydrolyzed 2-ketoarginine with a relative rate of about 0.7% of that toward 4-guanidinobutyrate. The Km for 2-ketoarginine was 33 mM.
-
McGill,Lindstrom
, p. 26,27 (1977)
-
-
Rampino,Svirbely
, p. 3534 (1939)
-
Catalytic Urea Synthesis from Ammonium Carbamate Using a Copper(II) Complex: A Combined Experimental and Theoretical Study
Dennis, Donovan,Ekmekci, Merve B.,Hanson, Danielle S.,Paripati, Amay,Wang, Yigui,Washburn, Erik,Xiao, Dequan,Zhou, Meng,Zhou, Xinrui
, p. 5573 - 5589 (2021/05/06)
The synthesis of urea fertilizer is currently the largest CO2 conversion process by volume in the industry. In this process, ammonium carbamate is an intermediate en route to urea formation. We determined that the tetraammineaquacopper(II) sulfate complex, [Cu(NH3)4(OH2)]SO4, catalyzed the formation of urea from ammonium carbamate in an aqueous solution. A urea yield of up to 18 ± 6% was obtained at 120 °C after 15 h and in a high-pressure metal reactor. No significant urea formed without the catalyst. The urea product was characterized by Fourier transform infrared (FT-IR), powder X-ray diffraction (PXRD), and quantitative 1H{13C} NMR analyses. The [Cu(NH3)4(OH2)]SO4 catalyst was then recovered at the end of the reaction in a 29% recovery yield, as verified by FT-IR, PXRD, and quantitative UV-vis spectroscopy. A precipitation method using CO2 was developed to recover and reuse 66 ± 3% of Cu(II). The catalysis mechanism was investigated by the density functional theory at the B3LYP/6-31G*? level with an SMD continuum solvent model. We determined that the [Cu(NH3)4]2+ complex is likely an effective catalyst structure. The study of the catalysis mechanism suggests that the coordinated carbamate with [Cu(NH3)4]2+ is likely the starting point of the catalyzed reaction, and carbamic acid can be involved as a transient intermediate that facilitates the removal of an OH group. Our work has paved the way for the rational design of catalysts for urea synthesis from the greenhouse gas CO2.
Electrochemical C-N coupling with perovskite hybrids toward efficient urea synthesis
Yuan, Menglei,Chen, Junwu,Bai, Yiling,Liu, Zhanjun,Zhang, Jingxian,Zhao, Tongkun,Shi, Qiaona,Li, Shuwei,Wang, Xi,Zhang, Guangjin
, p. 6048 - 6058 (2021/05/14)
Electrocatalytic C-N coupling reaction by co-activation of both N2 and CO2 molecules under ambient conditions to synthesize valuable urea opens a new avenue for sustainable development, while the actual catalytic activity is limited by poor adsorption and coupling capability of gas molecules on the catalyst surface. Herein, theoretical calculation predicts that the well-developed built-in electric field in perovskite hetero-structured BiFeO3/BiVO4 hybrids can accelerate the local charge redistribution and thus promote the targeted adsorption and activation of inert N2 and CO2 molecules on the generated local electrophilic and nucleophilic regions. Thus, a BiFeO3/BiVO4 heterojunction is designed and synthesized, which delivers a urea yield rate of 4.94 mmol h-1 g-1 with a faradaic efficiency of 17.18% at -0.4 V vs. RHE in 0.1 M KHCO3, outperforming the highest values reported as far. The comprehensive analysis further confirms that the local charge redistribution in the heterojunction effectively suppresses CO poisoning and the formation of the endothermic ?NNH intermediate, which thus guarantees the exothermic coupling of ?NN? intermediates with the generated CO via C-N coupling reactions to form the urea precursor ?NCON? intermediate. This work opens a new avenue for effective electrocatalytic C-N coupling under ambient conditions.
Unveiling Electrochemical Urea Synthesis by Co-Activation of CO2 and N2 with Mott–Schottky Heterostructure Catalysts
Yuan, Menglei,Chen, Junwu,Bai, Yiling,Liu, Zhanjun,Zhang, Jingxian,Zhao, Tongkun,Wang, Qin,Li, Shuwei,He, Hongyan,Zhang, Guangjin
, p. 10910 - 10918 (2021/04/19)
Electrocatalytic C?N bond coupling to convert CO2 and N2 molecules into urea under ambient conditions is a promising alternative to harsh industrial processes. However, the adsorption and activation of inert gas molecules and then the driving of the C–N coupling reaction is energetically challenging. Herein, novel Mott–Schottky Bi-BiVO4 heterostructures are described that realize a remarkable urea yield rate of 5.91 mmol h?1 g?1 and a Faradaic efficiency of 12.55 % at ?0.4 V vs. RHE. Comprehensive analysis confirms the emerging space–charge region in the heterostructure interface not only facilitates the targeted adsorption and activation of CO2 and N2 molecules on the generated local nucleophilic and electrophilic regions, but also effectively suppresses CO poisoning and the formation of endothermic *NNH intermediates. This guarantees the desired exothermic coupling of *N=N* intermediates and generated CO to form the urea precursor, *NCON*.
KV3 MODULATORS
-
Page/Page column 49-50, (2021/08/14)
A compound of formula (I) and related aspects.