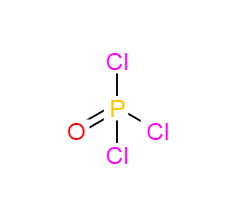
Phosphorus oxychloride literature
On the Reaction of Chlorine Nitrate ClONO2 with PCl3, AsCl3, SbCl3 and AsCl4+AsF6-
Minkwitz, Rolf,Hertel, Thomas,Meier, Ralf
, p. 1064 - 1066 (1996)
The reaction of ClONO2 with PCl3 yields POCl3 and NO2+NO3-. With AsCl3 chlorine nitrate forms a compound of the analytical composition AsCl2(NO3)3 , in contrast to SbCl3, which does not react with ClONO2. The reaction of ClONO2 with AsCl4+AsF6- was reported to yield As(ONO2)4+AsF6-. We found no reaction below 273 K. Reaction at 293 K yields NO2+AsF6- as solid product. SbCl5 does react, but the reaction yields no reproduceable products.
OXIDATION OF PHOSPHORUS(III) HALIDES BY RED PHOTOLYSIS OF OZONE COMPLEXES IN SOLID ARGON
Moores, Brian W.,Andrews, Lester
, p. 1902 - 1907 (1989)
PCl3-O3 and PBr3-O3 complexes in solid argon photolyze to give phosphoryl halides with red visible radiation that has no effect on isolated ozone.These observations and similar results for PH3-O3 and P4-O3 complexes show that the complex markedly increases the cross section for red photodissociation of ozone and suggest that this increase is due to the complex effectively lowering the barrier to dissociation by providing a strongly exothermic dissociation-recombination process.
CRYSTAL STRUCTURES OF A CESIUM IRON(III) PHOSPHATE, Cs3Fe4(PO4)5, AND A CESIUM IRON(III) OXYPHOSPHATE, Cs7Fe7(PO4)8O2.
Andrews-Allen,Robinson
, p. 88 - 97 (1988)
The reaction of FePO//4 with molten CsCl produces Cs//3Fe//4(PO//4)//5, Cs//7Fe//7(PO//4)//8O//2, or Fe//2O//3, depending on the length of the reaction. The crystal structure of Cs//3Fe//4(PO//4)//5 consists of an interlocking network of corner-sharing phosphate tetrahedra and iron trigonal bipyramids with cesium atoms situated in tunnels. The Cs//7Fe//7(PO//4)//8O//2 structure can be described as a three-dimensional array containing linked phosphate tetrahedra and iron octahedra, trigonal bipyramids, and square pyramids. In addition to the phosphate oxygen atoms, three of the iron polyhedra share a common oxide ion. The cesium atoms are located in tunnels.
Reaction kinetics of PO2Cl-, PO2Cl 2-, POCl2- and POCl3 - with O2 and O3 from 163 to 400 K
Fernandez, Abel I.,Midey, Anthony J.,Miller, Thomas M.,Viggiano
, p. 9120 - 9125 (2004)
Rate constants and product ion branching fractions for the gas-phase reactions of O2 and O3 with the anions (a) PO 2Cl-, (b) POCl3-, (c) POCl 2-, and (d) PO2Cl2- were measured in a selected-ion flow tube (SIFT). The kinetics were measured at temperatures of 163-400 K and a He pressure of 0.4 Torr. Only PO 2Cl- reacts with O2 to a measurable extent, having k(163-400 K) = 1.1 × 10-8(T/K)-1.0 cm 3 molecule-1 s-1, while O3 reacts with all of the anions except PO2Cl2-. The fitted rate constant expressions for the O3 reaction with anions a-c are as follows: ka(163-400 K) = 3.5 × 10-6(T/K) -1.6, kb(163-400 K) = 4.0 × 10 -7(T/K)-1.2, and kc(163-400 K) = 3.7 × 10-7(T/K)-1.4 cm3 molecule-1 s -1. Calculations were performed at the G3 level of theory to obtain optimized geometries, energies, and electron affinities (EAs) of the reactant and product species, as well as to determine the reaction thermochemistry to help understand the experimental results. The POxCly - anions that have lower electron binding energies (eBE) and higher spin multiplicities are more reactive. The doublets are more labile than the singlets. How the extra electron density is distributed in the anion does not predict the observed reactivity of the ion. The reactions of PO 2Cl- with O2 and O3 yield predominantly PO3- and PO4-. The reaction of POCl2- with O3 yields mostly Cl- and PO2Cl2-, while the POCl 3- reaction with O3 yields mostly O 3- and PO2Cl2-.
Reaction of P(III) chlorides with aldehydes: III. Reaction of primary intermediates with oxidants and chlorinating agents
Gazizov,Khairullin,Karimova
, (2014)
Primary intermediates of P(III) chlorides reaction with aldehydes have been converted into the corresponding phosphates by treating with oxidants: dimethylsulfoxide and tert-butyl hypochlorite. In reactions of the intermediates with chlorinating agents (P
-
Haszeldine,Iserson
, p. 5801,5803 (1957)
-
Cross-linked poly(4-vinylpyridine-N-oxide) as a polymer-supported oxygen atom transfer reagent
Bauer, Anna M.,Ramey, Erin E.,Oberle, Kjersti G.,Fata, Gretchen A.,Hutchison, Chloe D.,Turlington, Christopher R.
, (2019)
Oxygen atom transfer (OAT) reagents are common in biological and industrial oxidation reactions. While many heterogeneous catalysts have been utilized in OAT reactions, heterogeneous OAT reagents have not been explored. Here, cross-linked poly(4-vinylpyridine-N-oxide), called x-PVP-N-oxide, was tested as a heterogeneous OAT reagent and its oxidation chemistry compared to its molecular counterpart, pyridine-N-oxide. The insoluble oxidant x-PVP-N-oxide demonstrated comparable reactivity to pyridine-N-oxide in direct oxidation reactions of phosphines and phosphites in acetonitrile, but x-PVP-N-oxide did not react in other solvents. The polymer backbone of x-PVP-N-oxide, however, allowed for easy filtering and recycling in sequential oxidation reactions. In addition, x-PVP-N-oxide was tested as the stoichiometric oxidant in a copper-catalyzed OAT reaction to α-diazo-benzeneacetic acid methyl ester. The heterogeneous oxidant was much less reactive than pyridine-N-oxide, indicating that interaction with the metal catalyst was challenging. These results demonstrated a proof-of-concept that recyclable, polymer-supported OAT reagents could be a viable OAT reagents in direct oxidation reactions without metal catalysts.
-
Bannard et al.
, p. 976,981 (1953)
-
An improved and efficient synthesis of pinene based bipyridyldiols and bipyridine
Boobalan, Ramalingam,Chen, Chinpiao
, p. 1930 - 1934 (2016)
An improved and efficient synthesis of pinene based two bipyridyldiols and bipyridine is reported. For the first time, the sealed tube-pressure reaction of pinene based pyridone with phosphoryl chloride produced an excellent yield (95%) of pinene based 2-chloropyridine, which renders synthesizing pinene based bipyridyldiols a highly inexpensive and high yielding process. Moreover, highly effective reaction condition was developed for homocoupling of chloropyridine with Ni(0) that afforded pinene based bipyridine in a high yield (84%). These newly demonstrated sealed tube-pressure chlorination and homocoupling reaction of chloropyridine afford extremely effect route for the synthesis of pinene based bipyridine.
Method for co-producing high-purity phosphorus oxychloride by alkyl phosphonic acid chloride monoalkyl ester
-
Paragraph 0022; 00213, (2019/11/20)
The invention discloses a method for co-producing high-purity phosphorus oxychloride by alkyl phosphonic acid monoalkyl ester, and the method comprises the following steps: (1) reacting phosphorus pentachloride and alkyl phosphonic acid dialkyl ester in a