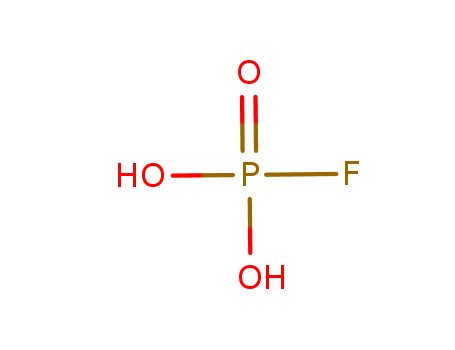
FLUOROPHOSPHORIC ACID literature
Initial stages of thermal decomposition of LiPF6-based lithium ion battery electrolytes by detailed Raman and NMR spectroscopy
Wilken, Susanne,Treskow, Marcel,Scheers, Johan,Johansson, Patrik,Jacobsson, Per
, p. 16359 - 16364 (2013/09/23)
Independent of the specific electrode chemistry, the state-of-the-art lithium ion battery electrolytes based on LiPF6 in organic solvents have a low thermal abuse tolerance and poor cycle life at elevated temperatures. We present here a detailed investigation of the initial stages of the thermal decomposition of LiPF6 in EC/DMC stored at 85 °C using Raman and NMR spectroscopy. During storage (up to 160 h), significant amounts of CO 2 are evolved, as detected in the Raman spectra. Time-resolved 1H, 31P, and 19F NMR spectra show the evolution of POF3, POF(OH)2, POF2(OCH2CH 2)nF, and POF2OMe as reactive decomposition products. Our unique 19F NMR approach, measuring while heating with both high energy and time resolution, allows for a first quantitative analysis of the evolved species and reveals several decomposition reactions during the first 30 min up to 72 h, where the rates of HF and POF2OMe formation are surprisingly linear. EC is found to be much less reactive compared to DMC. All information is used in the formulation of an updated decomposition pathway chart for LiPF6 based electrolytes. The Royal Society of Chemistry 2013.
Formation of monofluorophosphate from fluoride in phosphoric acid - Water and phosphoric acid - sulfuric acid - water mixtures
Newman, Kenneth E.,Ortlieb, Raymond E.,Pawlik, Nicole,Reedyk, Jason
, p. 346 - 351 (2008/03/13)
When dissolved in concentrated phosphoric acid, sodium fluoride reacts rapidly to form monofluorophosphate. In less concentrated acid, the reaction does not proceed to completion, and the reaction kinetics become very much slower. The equilibrium and rate
Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries
Campion, Christopher L.,Li, Wentao,Lucht, Brett L.
, p. A2327-A2334 (2008/10/09)
The thermal decomposition of lithium-ion battery electrolytes 1.0 M LiPF6 in one or more carbonate solvents has been investigated. Electrolytes containing diethyl carbonate (DEC), ethylene carbonate (EC), a 1:1 mixture of EC/dimethyl carbonate (DMC), and a 1:1:1 mixture EC/DMC/DEC have been investigated by multinuclear nuclear magnetic spectroscopy, gas chromatography with mass selective detection, and size exclusion chromatography. Thermal decomposition affords products including: carbon dioxide (CO 2), ethylene (CH2CH2), dialkylethers (R 2O), alkyl fluorides (RF), phosphorus oxyfiuoride (OPF3), fluorophosphates [OPF2OR, OPF(OR)2], fluorophosporic acids [OPF2OH, OPF(OH)2], and oligoethylene oxides. The mechanism of decomposition is similar in all LiPF6/carbonate electrolytes. Trace protic impurities lead to generation of OPF2OR, which autocatalytically decomposes LiPF6 and carbonates. The presence of DEC leads to the generation of ethylene, while the presnce of EC leads to the generation of capped oligothylene oxides [OPF2(OCH 2CH2)nF].