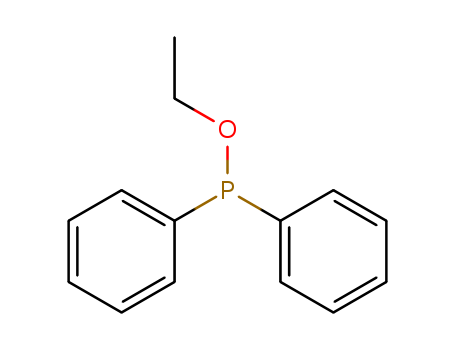
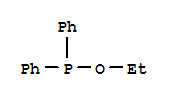Ethyl diphenylphosphinite literature
Reactions of Ferrocenium Hexafluorophosphate with P?OR Nucleophiles Give Ring C?H Functionalization or Ring Replacement Products Depending on the Phosphorus Reagent
Chamkin, Aleksandr A.,Krivykh, Vasily V.,Kreindlin, Arkady Z.,Dolgushin, Fedor M.,Ustynyuk, Nikolai A.
, p. 1601 - 1610 (2021/04/16)
Ferrocenium hexafluorophosphate reacts with different P?OR nucleophiles (PR3) in CH2Cl2 at room temperature to give either half-sandwich complexes [CpFe(PR3)3](PF6) (PR3=P(OMe)3, P(OEt)3, PhP(OMe)2) or ferrocenylphosphonium salts [CpFe(C5H4PR3)](PF6) (PR3=iPr2P(OMe), iPr2P(OEt)). Mixtures of both products are formed for some other nucleophiles (PR3=Ph2P(OMe), Ph2P(OEt), PhP(OiPr)2). The mechanism of the former reaction was established using DFT calculations. This reaction pathway is especially characteristic of π-acceptor nucleophiles, which is presumably explained by their ability to stabilize the 19e intermediates. The result of the reaction with tertiary phosphines, aminophosphines, and P?OR nucleophiles can be reliably predicted based on the values of the Tolman electronic parameter (below 2070 cm?1 – only ferrocenylphosphonium salt, in between 2073 cm?1 and 2080 cm?1 – only half-sandwich complex, and in the range from 2070 cm?1 to 2073 cm?1 – mixtures of both products).
Monoacylphosphine oxides with substituents in the phosphonyl moiety as Norrish I photoinitiators: Synthesis, photoinitiattion properties and mechanism
Duan, Haodong,Gao, Jun,Han, Yuxi,Leng, Kangwei,Li, Qianmin,Liu, Dayong,Wang, Zhongwei,Xu, Xiaolei,Yu, Qing
, (2021/09/07)
In order to study the effect of the substituents in the phosphonyl moiety of monoacylphosphine oxide (MAPO) on its stability and initiation performance, (2,4,6-trimethylphenyl)(phenyl)(benzoyl)phosphine oxide (TMBPO), (4-tolyl)(phenyl)(2,4,6-trimethylbenzoyl)phosphine oxide (4-MTPO) and (2,4-xylyl)(phenyl)(2,4,6-trimethylbenzoyl)phosphine oxide (2,4-DMTPO) were designed and prepared. Studies on TMBPO showed that the introduction of methyl groups into the phosphonyl moiety of MAPO significantly enhanced its stability and light absorption abilities. The photopolymerization of trimethylolpropane triacrylate (TMPTA) showed that the initiation efficiency of 4-MTPO and 2,4-DMTPO were higher than that of TPO, regardless of whether it was initiated upon LED at 385 nm or 420 nm. In addition, the migration rates of 4-MTPO and 2,4-DMTPO in cured TMPTA were approximately 1/2 and 1/4 that of TPO, respectively.
Silver-Catalyzed Regioselective Phosphorylation of para-Quinone Methides with P(III)-Nucleophiles
Liu, Yu,Tang, Ke-Wen,Wong, Wai-Yeung,Xie, Jun,Xiong, Biquan,Xu, Shipan,Xu, Weifeng
, p. 14983 - 15003 (2021/11/12)
A simple and efficient method for the silver-catalyzed regioselective phosphorylation of para-quinone methides (p-QMs) with P(III)-nucleophiles (P(OR)3, ArP(OR)2, Ar2P-OR) has been established via Michaelis-Arbuzov-type reaction. A broad range of P(III)-nucleophiles and para-quinone methides are well tolerated under the mild conditions, giving the expected diarylmethyl-substituted organophosphorus compounds with good to excellent yields. Moreover, a series of corresponding enantiomers can be obtained by employing dialkyl arylphosphonite (ArP(OR)2) as substrates. The control experiments and 31P NMR tracking experiments were also performed to gain insights for the plausible reaction mechanism. This protocol may have significant implications for the formation of C(sp3)-P bonds in Michaelis-Arbuzov-type reactions.
Preparation method of material compound
-
Paragraph 0050-0055; 0064-0065, (2021/04/10)
The invention provides a preparation method of a diarylphosphonate compound, which comprises the following steps: in a protective gas atmosphere, taking diarylphosphonic acid and halogenated alkane as raw materials, taking heteropoly acid as a catalyst and taking an organic solvent as a solvent, and conducting reacting to obtain the diarylphosphonate compound. According to the preparation method disclosed by the invention, only an extremely small amount of catalyst is needed, the reaction temperature is relatively mild, the reaction time can be obviously shortened, the yield and purity are relatively high, and an unexpected technical effect is achieved.