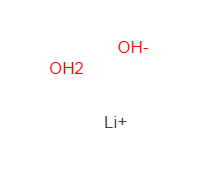
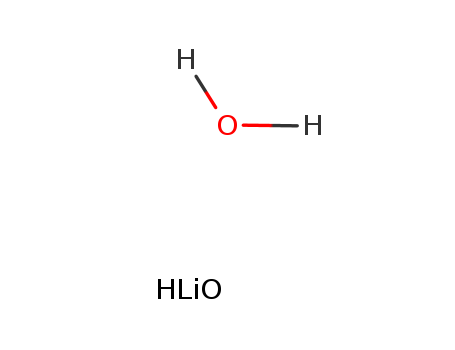
Lithium hydroxide monohydrate literature
Preparation of high-purity lithium hydroxide monohydrate from technical-grade lithium carbonate by membrane electrolysis
Ryabtsev,Nemkov,Kotsupalo,Serikova
, p. 1108 - 1116 (2004)
A scheme for preparing high-purity lithium hydroxide monohydrate from technical-grade lithium carbonate is suggested.
Local crystal structure around manganese in new potassium-based nanocrystalline manganese oxyiodide
Hwang, Seong-Ju,Kwon, Chai-Won,Portier, Josik,Campet, Guy,Park, Hyo-Suk,Choy, Jin-Ho,Huong, Pham V.,Yoshimura, Masahiro,Kakihana, Masato
, p. 4053 - 4060 (2002)
A new nanocrystalline potassium-based lithium manganese oxyiodide has been prepared by using Chimie Douce route at room temperature. According to the electrochemical measurements, this nanocrystalline sample shows a large initial capacity up to a??340 mAh/g at a constant current density of 0.2 mA/cm2, which is much larger than that of sodium-based homologue. The X-ray diffraction analysis demonstrates that the amorphous character of the nanocrystalline compounds is maintained before and after chemical lithiation reaction. The local crystal structure around manganese in these materials has been determined by performing the combinative micro-Raman and X-ray absorption spectroscopy. From the Mn K-edge X-ray absorption near-edge structure and micro-Raman results, it becomes certain that manganese ions are stabilized in the rhombohedral layered lattice consisting of edge-shared MnO6 octahedra, and the crystal symmetry is changed into a monoclinic symmetry upon reaction with n-BuLi. The Mn K-edge extended X-ray fine structure analysis reveals that the structural distortion caused by lithiation process is less significant for these nanocrystalline compounds than for the spinel lithium manganate. In this context, the great discharge capacity of the nanocrystalline materials is attributable for the pillaring effect of larger alkali metal ion than lithium ion, providing an expanded interlayer space available for Li insertion. In addition, the I LI-edge X-ray absorption near-edge structure results presented here make it clear that iodine is stabilized as iodate species on the grain boundary or the surface of the nanocrystalline manganese oxyiodide, which helps to maintain the nanocrystalline nature of the present materials before and after Li insertion.
Scalable integration of Li5FeO4 towards robust, high-performance Lithium-ion hybrid capacitors
Park, Min-Sik,Lim, Young-Geun,Hwang, Soo Min,Kim, Jung Ho,Kim, Jeom-Soo,Dou, Shi Xue,Cho, Jaephil,Kim, Young-Jun
, p. 3138 - 3144 (2014)
Lithium-ion hybrid capacitors have attracted great interest due to their high specific energy relative to conventional electrical double-layer capacitors. Nevertheless, the safety issue still remains a drawback for lithium-ion capacitors in practical operational environments because of the use of metallic lithium. Herein, single-phase Li5FeO4 with an antifluorite structure that acts as an alternative lithium source (instead of metallic lithium) is employed and its potential use for lithium-ion capacitors is verified. Abundant Li+ amounts can be extracted from Li5FeO4 incorporated in the positive electrode and efficiently doped into the negative electrode during the first electrochemical charging. After the first Li+ extraction, Li+ does not return to the Li5FeO4 host structure and is steadily involved in the electrochemical reactions of the negative electrode during subsequent cycling. Various electrochemical and structural analyses support its superior characteristics for use as a promising lithium source. This versatile approach can yield a sufficient Li+-doping efficiency of >90% and improved safety as a result of the removal of metallic lithium from the cell.
Synthesis, short-range structure, and electrochemical properties of new phases in the Li-Mn-N-0 system
Cabana, Jordi,Dupre, Nicolas,Gillot, Frederic,Chadwick, Alan V.,Grey, Clare P.,Palacin, M. Rosa
, p. 5141 - 5153 (2009)
A crystal-chemical exploration of part of the Li - Mn - N - O system was carried out. Several samples were synthesized using Li3N, Mn xN and Li2O and characterized with chemical analysis, XRD, XAS, and NMR. An increase in
The effect of 3D carbon nanoadditives on lithium hydroxide monohydrate based composite materials for highly efficient low temperature thermochemical heat storage
Li, Shijie,Huang, Hongyu,Li, Jun,Kobayashi, Noriyuki,Osaka, Yugo,He, Zhaohong,Yuan, Haoran
, p. 8199 - 8208 (2018/03/09)
Lithium hydroxide monohydrate based thermochemical heat storage materials were modified with in situ formed 3D-nickel-carbon nanotubes (Ni-CNTs). The nanoscale (5-15 nm) LiOH·H2O particles were well dispersed in the composite formed with Ni-CNTs. These composite materials exhibited improved heat storage capacity, thermal conductivity, and hydration rate owing to hydrogen bonding between H2O and hydrophilic groups on the surface of Ni-CNTs, as concluded from combined results of in situ DRIFT spectroscopy and heat storage performance test. The introduction of 3D-carbon nanomaterials leads to a considerable decrease in the activation energy for the thermochemical reaction process. This phenomenon is probably due to Ni-CNTs providing an efficient hydrophilic reaction interface and exhibiting a surface effect on the hydration reaction. Among the thermochemical materials, Ni-CNTs-LiOH·H2O-1 showed the lowest activation energy (23.3 kJ mol-1), the highest thermal conductivity (3.78 W m-1 K-1) and the highest heat storage density (3935 kJ kg-1), which is 5.9 times higher than that of pure lithium hydroxide after the same hydration time. The heat storage density and the thermal conductivity of Ni-CNTs-LiOH·H2O are much higher than 1D MWCNTs and 2D graphene oxide modified LiOH·H2O. The selection of 3D carbon nanoadditives that formed part of the chemical heat storage materials is a very efficient way to enhance comprehensive performance of heat storage activity components.
Extended Chemical Flexibility of Cubic Anti-Perovskite Lithium Battery Cathode Materials
Lai, Kwing To,Antonyshyn, Iryna,Prots, Yurii,Valldor, Martin
supporting information, p. 13296 - 13299 (2018/10/31)
Novel bichalcogenides with the general composition (Li2TM)ChO (TM = Mn, Co; Ch = S, Se) were synthesized by single-step solid-state reactions. These compounds possess cubic anti-perovskite crystal structure with Pm3m symmetry; TM and Li are disordered on the crystallographic site 3c. According to Goldschmidt tolerance factor calculations, the available space at the 3c site is too large for Li+ and TM2+ ions. As cathode materials, all title compounds perform less prominent in lithium-ion battery setups in comparison to the already known TM = Fe homologue; e.g., (Li2Co)SO has a charge density of about 70 mAh g-1 at a low charge rate. Nevertheless, the title compounds extend the chemical flexibility of the anti-perovskites, revealing their outstanding chemical optimization potential as lithium battery cathode material.
Anti-Perovskite Li-Battery Cathode Materials
Lai, Kwing To,Antonyshyn, Iryna,Prots, Yurii,Valldor, Martin
supporting information, p. 9645 - 9649 (2017/07/24)
Through single-step solid-state reactions, a series of novel bichalcogenides with the general composition (Li2Fe)ChO (Ch = S, Se, Te) are successfully synthesized. (Li2Fe)ChO (Ch = S, Se) possess cubic anti-perovskite crystal structures, where Fe and Li are completely disordered on a common crystallographic site (3c). According to Goldschmidt calculations, Li+ and Fe2+ are too small for their common atomic position and exhibit large thermal displacements in the crystal structure models, implying high cation mobility. Both compounds (Li2Fe)ChO (Ch = S, Se) were tested as cathode materials against graphite anodes (single cells); They perform outstandingly at very high charge rates (270 mA g-1, 80 cycles) and, at a charge rate of 30 mA g-1, exhibit charge capacities of about 120 mA h g-1. Compared to highly optimized Li1-xCoO2 cathode materials, these novel anti-perovskites are easily produced at cost reductions by up to 95% and, yet, possess a relative specific charge capacity of 75%. Moreover, these iron-based anti-perovskites are comparatively friendly to the environment and (Li2Fe)ChO (Ch = S, Se) melt congruently; the latter is advantageous for manufacturing pure materials in large amounts.