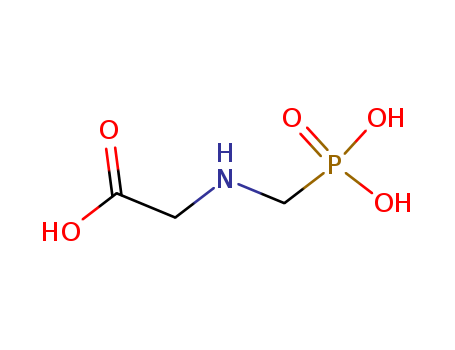
Glyphosate literature
Chemoenzymic synthesis of N-(Phosphonomethyl)glycine
Gavagan, John E.,Fager, Susan K.,Seip, John E.,Clark, Dawn S.,Payne, Mark S.,Anton, David L.,DiCosimo, Robert
, p. 5419 - 5427 (1997)
Permeabilized, metabolically-inactive transformants of the methylotrophic yeasts Hansenula polymorpha and Pichia pastoris which contain significant quantities of the enzymes spinach glycolate oxidase ((S)-2- hydroxyacid oxidase, EC 1.1.3.15), Saccharomyces cerevisiae catalase T (EC 1.11.1.6), and endogenous catalase have been used as catalysts for the oxidation of glycolic acid by oxygen to produce glyoxylic acid in aqueous mixtures containing (aminomethyl)phosphonic acid. After separation and recovery of the microbial catalyst from the oxidation product mixture for reuse, the resulting solution of glyoxylic acid and (aminomethyl)phosphonic acid was subsequently hydrogenated with a palladium/carbon catalyst to produce N-(phosphonomethyl)glycine (glyphosate), a broad-spectrum, postemergent herbicide. Complete conversion of (aminomethyl)phosphonic acid in the hydrogenation allowed the use of a simple acid precipitation for isolation of the N(phosphonomethyl)glycine from the hydrogenation product mixture in high purity and yield.
Electron-Transfer Agents in Metal-Catalyzed Dioxygen Oxidations: Effective Catalysts for the Interception and Oxidation of Carbon Radicals
Riley, Dennis P.,Fields, Donald L.
, p. 1881 - 1882 (1992)
-
X-RAY STRUCTURAL STUDY OF ORGANIC LIGANDS OF THE COMPLEXONE TYPE. III. CRYSTAL AND MOLECULAR STRUCTURE OF PHOSPHONOMETHYLGLYCINE AND IMINODIACETIC-MONOMETHYLPHOSPHONIC ACID
Shkol'nikova, L. M.,Porai-Koshits, M. A.,Dyatlova, N. M.,Yaroshenko, G. F.,Rudomino, M. V.,Kolova, E. K.
, p. 737 - 746 (1982)
An x-ray structural study of phosphonomethylglycine (I) and iminodiacetic-monomethylphosphonic acid (II) has been carried out (diffractometer, direct method, anisotropic method of least squares, R = 0.035 and 0.050, RW = 0.040 and 0.052 from 1712 and 1113 reflections for compounds I and II respectively).The crystals of compound I are monoclinic and those of compound II triclinic; I: a = 8.681, b = 7.981, c = 9.893 Angstroem, β = 105.77 deg, dcalc = 1.702 g/nm3, Z = 4, space group P21/c; II: a = 5.590, b = 7.422, c = 10.648 Angstroem, α = 93.12, β = 95.03, γ = 90.40 deg, dcalc = 1.716 g/cm3, Z = 2, space group <*>.The geometric parameters of the molecules of compounds I and II are similar.Structural proof has been obtained for the first time to show that the nitrogen atom is protonated by the proton of the phosphonic acid group, and not the carboxyl group, for the Complexones I and II, containing comparing functional groups, in the crystalline state.Complexone I is obtained from Complexone II by removing one methylcarboxyl group and replacing it by a hydrogen atom.The result of this process is a change in compound I from the conformation of the molecule of compound II, directed towards the stabilization of a sterically favorable system of hydrogen bonds (HB), responsible for the similar structural motifs in the crystals of compounds I and II.This system includes HB O -H...O and N -H...O, forming dimeric ribbons, networks, and three-dimensional frameworks.In compound II, weak intramolecular HB are formed, leading to the formation of H-rings.
Method for synthesizing glyphosate
-
Paragraph 0038; 0042; 0045; 0049; 0056; 0052; 0059; 0062, (2019/07/10)
The invention provides a method for synthesizing glyphosate. The method comprises the following steps: firstly, reacting a compound with a structure shown in a formula (I), paraformaldehyde, an organic solvent, water and a catalyst in a mixed gas atmosphere of carbon monoxide and hydrogen to obtain a compound with a structure shown in a formula (II); then, mixing and hydrolyzing the compound withthe structure shown in the formula (II) and concentrated hydrochloric acid to obtain the glyphosate, specifically, the compound with the structure shown in the formula (I) is used as a reaction raw material to prepare the glyphosate, the source of the reaction raw material is wide, and dimethyl phosphite is not needed to be synthesized as a reaction raw material to prepare the compound with the structure shown in the formula (I), but only phosphorous acid or even phosphorus trichloride can be directly used instead; a byproduct acetic acid prepared in the preparation process of the glyphosate can be used for preparing acetamide for recycling; and compared with an existing method for preparing glyphosate, the method has the advantages that the raw material utilization rate is higher, the process route is simplified, the generated waste liquid is less, and the method has good industrial application prospect.
NOXIOUS ARTHROPOD CONTROL AGENT CONTAINING AMIDE COMPOUND
-
, (2017/08/26)
An object of the present invention is to provide a compound having the controlling activity on a noxious arthropod, and a noxious arthropod controlling agent containing an amide compound of formula (I): wherein X represents a nitrogen atom or a CH group, p represents 0 or 1, A represents a tetrahydrofuranyl group or the like, R1, R2, R3, R4, R5, R6 and R7 represent a hydrogen atom or the like, n represents 1 or 2, Y represents an oxygen atom or the like, m represents any integer of 0 to 7, and Q represents a C1-8 chain hydrocarbon group optionally having a phenyl group or the like, has the excellent noxious arthropod controlling effect.
Glyphosate production method
-
Paragraph 0030-0053, (2017/08/28)
The invention discloses a glyphosate production method. According to the glyphosate production method, formaldehyde, phosphorous acid, glycine, hydrochloric acid and an oxidizing agent are adopted as main raw materials; the formaldehyde and the glycine are subjected to a reaction in a hydrochloric acid aqueous solution to generate dihydroxymethyl glycine, the phosphorous acid is added to the hydrochloric acid aqueous solution of the dihydroxymethyl glycine to generate N-hydroxymethyl glyphosate, hydrogen chloride is removing through pressure reducing removing, the N-hydroxymethyl glyphosate is subjected to catalytic oxidation in an aqueous solution by using a catalyst and an oxidizing agent to obtain glyphosate and formic acid, the generated formic acid is further oxidized into carbon dioxide and water, and due to the low solubility of the glyphosate at the low temperature, the glyphosate is separated from the reaction system by using a cooling crystallization method and is re-crystallized to obtain the raw drug glyphosate. According to the present invention, the synthesis method has advantages of simple process, easy operation, no use of the triethylamine catalyst, low cost, high product yield and high product purity, and the obtained glyphosate can be used as the weeding agent in agriculture and forestry, and has good application prospect.
Preparing method for glyphosate
-
Paragraph 0028; 0032; 0033, (2017/04/05)
The invention relates to a preparing method for glyphosate and belongs to the technical field of chemical engineering. The preparing method specifically comprises the four steps of synthesis of iminodiacetic acid, preparation of pmida, preparation of glyphosate and solid and liquid separation. According to the preparing method, firstly, precious metal silver is loaded on titanium dioxide, the surface nature of titanium dioxide is influenced, electron distribution is changed, and catalytic activity is improved; then, titanium dioxide loaded with silver is loaded into activated carbon and irradiated with an ultraviolet source. The selectivity of glyphosate is greatly improved, the oxidization process of pmida is greatly shortened, the yield of glyphosate is increased to 96.3%, and the product purity can reach 97.6%; besides, the content of methyl aldehyde is low, fewer products are generated, and the requirement of the nation for environment friendliness is met.