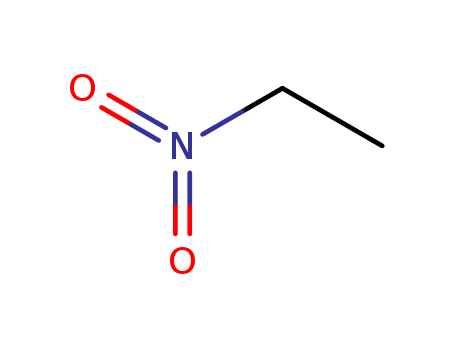
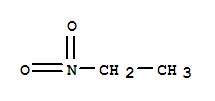Nitroethane literature
-
McCombie,Saunders,Wild
, p. 24 (1944)
-
Formation of positive and negative ions in CH3NO2
Jiao,DeJoseph Jr.,Garscadden
, p. 9040 - 9044 (2003)
Absolute dissociative ionization cross-sections from threshold to 200 eV have been measured using Fourier transform mass spectrometry (FTMS). In the production of positive ions by electron impact ionization, 13 ions are detected, including the parent ion
IR Spectroscopy of the Gas Phase Formed after Interaction between CH3I and Ag-Containing Sorbents Based on Silica Gel
Krapukhin, V. B.,Kulyukhin, S. A.,Rumer, I. A.
, p. 465 - 470 (2020/04/17)
Abstract: IR spectra of the gas phase formed during the interaction between gaseous CH3I and SiO2-based granular sorbents containing various silver compounds are studied. It is established that the main gaseous products formed by the
METHODS FOR FUNCTIONALIZATION HYDROCARBONS
-
Page/Page column 0158; 0163; 0182, (2020/09/27)
In one aspect, the disclosure relates to a method for functionalizing hydrocarbons. In a further aspect, the method involves heating a hydrocarbon with a composition having an acid and an oxidant. In other aspects, the composition can further include an iodine-based compound and/or a compound having formula AaXn. In any of these aspects, the oxidant can be regenerated in situ or in a separate regeneration step. Also disclosed are functionalized hydrocarbons produced by the disclosed method. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present disclosure.
Green synthesis of low-carbon chain nitroalkanes via a novel tandem reaction of ketones catalyzed by TS-1
Chu, Qingyan,He, Guangke,Xi, Yang,Wang, Ping,Yu, Haoxuan,Liu, Rui,Zhu, Hongjun
, p. 46 - 50 (2018/02/09)
A green and efficient one-pot method has been developed for the synthesis of low-carbon chain nitroalkanes via a novel TS-1 catalyzed tandem oxidation of ketones with H2O2 and NH3. The tandem reaction including ammoxidation, oximation and oxidation of oximes, afforded up to 88% yield and 98% chemo-selectivity requiring only 90 min, at 70 °C and atmospheric pressure. Moreover, this method was even amenable to 100-fold scale-up without loss of chemical efficiency with 87% yield, represents a significant advance towards industrial production of nitroalkanes. Furthermore, the plausible mechanism of TS-1 catalyzed tandem oxidation of ketones to prepare nitroalkanes was proposed.
Green synthesis method for preparing nitroalkanes by oxime oxidation
-
Paragraph 0049; 0050, (2017/08/29)
The invention belongs to the field of organic chemical industries, and provides a green synthesis method for preparing nitroalkanes by oxime oxidation. At the temperature of 55 to 120 DEG C and under the pressure of 0 to 1.0 MPa, oxime, a solvent and hydrogen peroxide are reacted for 20 to 200min in the presence of certain amounts of nanoporous skeleton metal hybrid catalysts and cocatalysts, a reaction liquid is subjected to membrane separation, the catalysts can be repeatedly used for more than 7 times, and distilled to obtain nitroalkane products, the purity of the products is not less than 99%, and the yield of the products is not less than 95%. Furthermore, the green synthesis method for preparing nitroalkanes by the oxime oxidation disclosed by the invention is a green synthesis method of nitroalkanes, and suitable for large-scale industrialized production.