Azithromycin dihydrate literature
A PROCESS FOR PREPARING 9-DEOXO-9A-AZA-9A-HOMOERYTHROMYCIN A
-
Page/Page column 12, (2008/06/13)
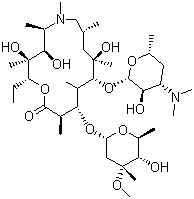
The invention relates to a process for preparing 9-Deoxo-9a-aza-9a- homoerythromycin A of formula (III), which is an intermediate in the preparation of Azithromycin dihydrate.
Process of preparing a crystalline azithromycin monohydrate
-
Page/Page column 5, (2008/06/13)
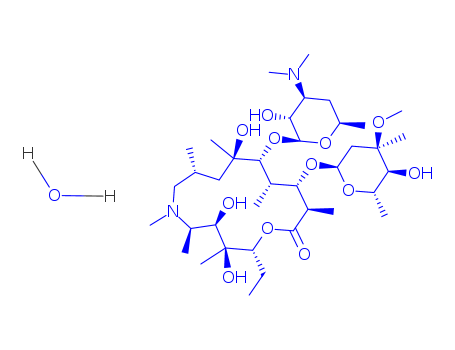
The present invention provides a process of preparing a crystalline azithromycin monohydrate. The process involves dissolving azithromycin in a solution containing ethanol, adding the dissolved azithromycin into water to precipitate the crystals, isolating and drying the precipitate to a water content of about 5% (w/w) to about 7% (w/w). The resulting azithromycin monohydrate is stable, exhibiting less than 2% degradation, and non-hydroscopic.
Stable non-dihydrate azithromycin oral suspensions
-
Page/Page column 16, (2008/06/13)
This invention relates to a powder for oral suspension, and an oral suspension made there from, which comprises non-dihydrate azithromycin and an azithromycin conversion stabilizing excipient, wherein said excipient reduces the conversion of the form of azithromycin, when placed in suspension, to another form of azithromycin. This invention further relates to a method for reducing the conversion of a form of non-dihydrate azithromycin, in an oral suspension, by adding a surface tension reducing excipient that reduces the surface tension of the aqueous vehicle. Furthermore, this invention relates to a method for reducing the conversion of a non-dihydrate azithromycin, in an unflavored oral suspension, by raising the viscosity of the oral suspension, and in a flavored oral suspension by lowering the viscosity of the oral suspension.
PROCESS FOR PREPARING NON-HYGROSCOPIC AZITHROMYCIN DIHYDRATE
-
Page 7, (2008/06/13)
A direct, single step process for preparing a semi-synthetic antibiotic azithromycin dihydrate (non-hygroscopic) is provided, by in situ reductive N-methylation of azaerythromycin A and subsequent crystallization from a mixture of acetone and water, preferably together with a catalytical quantity of a base such as liquor ammonia, which comprises: a) reacting azaerythromycin A with formic acid and formaldehyde in an organic solvent medium, to form a reaction mass comprising N-methylated azaerythromycin A (i.e. azithromycin); b) adding aqueous alkali solution to the reaction mass to form an aqueous phase and an organic phase; c) (i) when the organic solvent medium is acetone, separating the aqueous phase from the acetone organic phase, and removing the aqueous phase; or (ii) when the organic solvent medium is a solvent other than acetone, separating the aqueous phase from the organic phase and removing the aqueous phase, optionally washing the separated organic phase with aqueous alkali solution, completely distilling off the solvent from the organic phase to leave a residue, and adding acetone to dissolve the residue and form an acetone organic phase; d) adding water, and optionally a base, to the acetone organic phase to form a mixture, and allowing crystals to form in the mixture; e) recovering the crystals from the mixture and optionally washing the crystals; f) drying the crystals to obtain non-hygroscopic azithromycin dihydrate, and the yield of the non-hygroscopic azithromycin dihydrate of a pharmaceutically acceptable quality is obtained in yields up to 78-82% w/w.