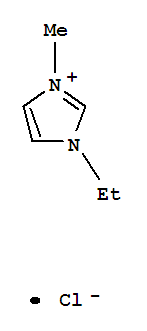
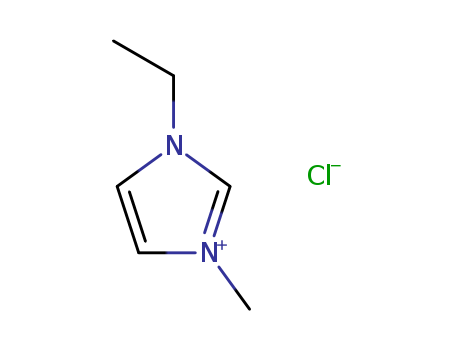
1-Ethyl-3-methylimidazolium chloride literature
Syntheses and physicochemical properties of new ionic liquids based on the hexafluorouranate anion
Kanatani, Takatsugu,Matsumoto, Kazuhiko,Hagiwara, Rika
, p. 714 - 715 (2009)
The first syntheses of a series of ionic liquids based on the hexafluorouranate(V) anion are described along with their physicochemical and electrochemical properties. The green roomtemperature ionic liquid, 1-ethyl-3-methylimidazolium hexafluorouranate (EMImUF6), exhibits a conductivity of 7.9 mS cm-1, viscosity of 59 cP, and electrochemical window of 2.3 V at 298 K. Copyright
Broensted Superacidity of HCl in a Liquid Chloroaluminate. AlCl3-1-Ethyl-3-methyl-1H-imidazolium Chloride
Smith, G. P.,Dworkin, A. S.,Pagni, R. M.,Zingg, S. P.
, p. 525 - 530 (1989)
The system HCl (0.1 - 1 atm)/AlCl3-EMIC (55.0 mol percent AlCl3)(EMIC = 1-ethyl-3-methyl-1H-imidazolium chloride) at 23 deg C is a Broensted superacid capable of protonating arenes to a degree similar to that of liquid HF at 0 deg C (H0=-15.1).Arenes used in this investigation were biphenyl (I), naphthalene (II), 9H-fluorene (III), chrysene (IV), 2-methylnaphthalene (V), mesitylene (VI), pentamethylbenzene (VII), hexamethylbenzene (VIII), anthracene (IX), and 9,10-dimethylanthracene (X).In both the chloroaluminate melt and HF I is a weak base while VIII-X are strong bases.In between these extremes the order of basicities in both media is II arene*H(1+) + 2AlCl4(1-) and is reversible.The degree of protonation was measured by optical absorption spectrophotometry.The arenes are stable in the liquid chloroaluminate for many hours, and their protonated forms (arenium ions) are stable for 1 h or more.A new procedure for the preparation of EMIC was developed that yields exceptionally clean AlCl3-EMIC melts with very low concentrations of protic and oxidizing impurities.
Preparation of nanocellulose using ionic liquids: 1-propyl-3-methylimidazolium chloride and 1-ethyl-3-methylimidazolium chloride
Babicka, Marta,Borysiak, S?awomir,Dwiecki, Krzysztof,Ratajczak, Izabela,Wo?niak, Magdalena
, (2020)
Cellulose nanocrystals were prepared using ionic liquids (ILs), 1-ethyl-3-methylimidazolium chloride [EMIM][Cl] and 1-propyl-3-methylimidazolium chloride [PMIM][Cl], from microcrystalline cellulose. The resultant samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The XRD results showed that nanocellulose obtained by treatment with both ILs preserved basic cellulose I structure, but crystallinity index of samples (except for Sigmacell treated with [EMIM][Cl]) was lower in comparison to the starting microcrystalline cellulose. The DLS results indicated noticeably smaller particle sizes of prepared cellulose for material treated with [PMIM][Cl] compared to cellulose samples hydrolyzed with [EMIM][Cl], which were prone to agglomeration. The obtained nanocellulose had a rod-like structure that was confirmed by electron microscopy analyses. Moreover, the results described in this paper indicate that cation type of ILs influences particle size and morphology of cellulose after treatment with ionic liquids.
Four fluoroborates ion liquid synthetic method of the (by machine translation)
-
Page/Page column 9-10, (2019/05/22)
The invention discloses a four-tetrafluoroborate ionic liquid synthetic method, comprises the following steps: the molar ratio of 1:1 - 7 of the quaternized substrate with the alkyl halide dispersed in a solvent, in the 30 - 50 °C lower, by UV-irradiation, the illumination intensity for 10 - 1000 W/m2 , Irradiation time is 8 - 12 is H, to obtain the quaternary ammonium salt solution; the quaternary ammonium salt solution with four fluoroborates mix, in the 35 - 45 °C lower, by UV-irradiation, the illumination intensity for 10 - 1000 W/m2 , Irradiation time is 16 - 20 the H, get mixed solution; filtering the mixed solution, to obtain the filtrate, the filtrate is heated and concentrated, to remove the solvent, to obtain four tetrafluoroborate ionic liquid. The invention by UV-irradiation and heating way to synthesize four tetrafluoroborate ionic liquid, yield and the reaction rate is high. (by machine translation)
Ionic Liquids Catalyzed Friedel–Crafts Alkylation of Substituted Benzenes with CCl4 Toward Trichloromethylarenes
Lyu, Xinyu,Wang, Wencheng,Sun, Yiqun,Zhao, Qian,Qiu, Tao
, p. 665 - 671 (2019/01/04)
Abstract: An ionic liquid catalyzed Friedel–Crafts alkylation reaction of substituted benzenes with CCl4 was developed. The reaction proceeded efficiently under mild conditions, gave corresponding trichloromethylarenes with diversity functional groups in moderate to good yields. The influence of Lewis acidity of ionic liquids on the conversion of the alkylation reaction has been investigated. Notably, the probable mechanism of this reaction has been proposed with the assistance of 27Al NMR spectroscopy. It was noteworthy that the predominance of [Al2Cl7]? species in EmimCl–AlCl3, N = 0.67 could be detected by 27Al NMR spectral analysis, and [AlCl4]? was generated at the beginning of reaction. Additionally, it was found that [AlCl4]? could be transformed into [Al2Cl7]? when the reaction finished. Some control experiments confirmed that the interaction between Lewis acidic species [Al2Cl7]? of the ionic liquid and CCl4 led to the change in speciation of aluminum during the alkylation reactions. Graphical Abstract: [Figure not available: see fulltext.].
Ionothermal synthesis, structures, properties of cobalt-1,4-benzenedicarboxylate metal-organic frameworks
Zhang, Zong-Hui,Xu, Ling,Jiao, Huan
, p. 217 - 222 (2016/04/06)
Eight kinds of 1-methyl-3-alkylimidazolium halide [RMI]X (R=ethyl (E), propyl (P), butyl (B) and amyl (A); MI = imidazolium; X= Cl-, I-) ionic liquids (ILs) were used as reaction media and obtained a series of 2D [RMI]2[Co3(BDC)3X2] frameworks through the ionothermal reactions of 1,4-benzenedicarboxylic acid (H2BDC) with Co(NO3)2·6H2O. The 2D [RMI]2[Co3(BDC)3X2] frameworks exhibit a same (3,6) topology network with [RMI]+ cations locating in the interlayer space. [RMI]+ cations play a template role in the structure constructions, whose influence combining with the effect of X- anions pass to the TG behaviors. The decomposition temperatures of the [RMI]2[Co3(BDC)3X2] frameworks decrease with the alkyl chains in [RMI]+ cations, and the compounds containing Cl- show higher thermal stabilities than those with I-. However, compounds 1-8 exhibit two similar broad emissions at ca. 380 and 390 nm, assigned to ILCT. The RMI+ templates and the X- anions do not exert their influence on the fluorescence.