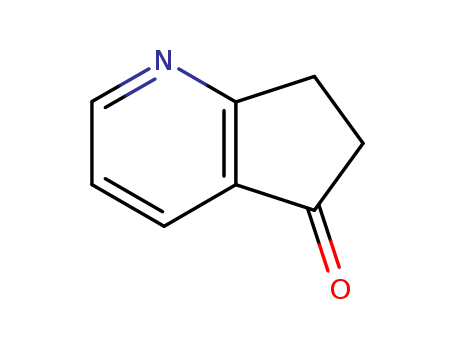
6,7-DIHYDRO-5H-1-PYRIDIN-5-ONE literature
The improved preparation of 7,8-dihydro-quinoline-5(6H)-one and 6,7- dihydro-5H-1-pyrindin-5-one
Huang, Yanhe,Hartmann, Rolf W.
, p. 1197 - 1200 (1998)
Compounds 7,8-dihydroquinoline-5(6H)-one (3) and 6,7-dihydro-5H-1- pyrindin-5-one (5) are formed by new methods from 1,3-diketone compound, ammonium acetate and 1,1,3,3-tetraethoxylpropane.
Selective Electrochemical Oxygenation of Alkylarenes to Carbonyls
Li, Xue,Bai, Fang,Liu, Chaogan,Ma, Xiaowei,Gu, Chengzhi,Dai, Bin
supporting information, p. 7445 - 7449 (2021/10/02)
An efficient electrochemical method for benzylic C(sp3)-H bond oxidation has been developed. A variety of methylarenes, methylheteroarenes, and benzylic (hetero)methylenes could be converted into the desired aryl aldehydes and aryl ketones in moderate to excellent yields in an undivided cell, using O2 as the oxygen source and lutidinium perchlorate as an electrolyte. On the basis of cyclic voltammetry studies, 18O labeling experiments, and radical trapping experiments, a possible single-electron transfer mechanism has been proposed for the electrooxidation reaction.
Preparation method of 6,7-dihydro-5H-cyclopentadieno[b] pyridine-5-one
-
Paragraph 0011-0014, (2019/12/29)
The invention provides a preparation method of 6,7-dihydro-5H-cyclopentadieno[b] pyridine-5-one, and the preparation method comprises the following steps: by using a compound (I) as a substrate, Fe <3 + > as a catalyst, a 50% hydrogen peroxide aqueous solution as an oxide and acetonitrile as a solvent, reacting at 50 DEG C for 24 hours to obtain a crude product, and carrying out column chromatography to obtain a pure compound (II). The method for preparing the compound (II) is mild in reaction condition, easy to purify, simple to operate and high in yield.
Electrochemical benzylic oxidation of C-H bonds
Marko, Jason A.,Durgham, Anthony,Bretz, Stacey Lowery,Liu, Wei
supporting information, p. 937 - 940 (2019/01/23)
Oxidized products have become increasingly valuable as building blocks for a wide variety of different processes and fine chemistry, especially in the benzylic position. We report herein a sustainable protocol for this transformation through C-H functionalization and is performed using electrochemistry as the main power source and tert-butyl hydroperoxide as the radical source for the C-H abstraction. The temperature conditions reported here do not increase above 50 °C and use an aqueous-based medium. A broad substrate scope is explored, along with bioactive molecules, to give comparable and increased product yields when compared to prior reported literature without the use of electrochemistry.
Iron-catalyzed oxidative functionalization of C(sp3)-H bonds under bromide-synergized mild conditions
Yu, Han,Zhao, Qixin,Wei, Zheyu,Wu, Zhikang,Li, Qi,Han, Sheng,Wei, Yongge
supporting information, p. 7840 - 7843 (2019/07/12)
An efficient oxidation and functionalization of C-H bonds with an inorganic-ligand supported iron catalyst and hydrogen peroxide to prepare the corresponding ketones was achieved using the bromide ion as a promoter. Preliminary mechanistic investigations indicated that the bromide ion can bind to FeMo6 to form a supramolecular species (FeMo6·2Br), which can effectively catalyze the reaction.