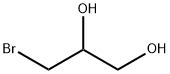
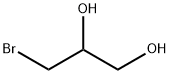
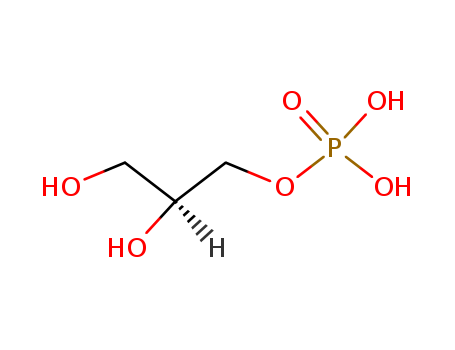
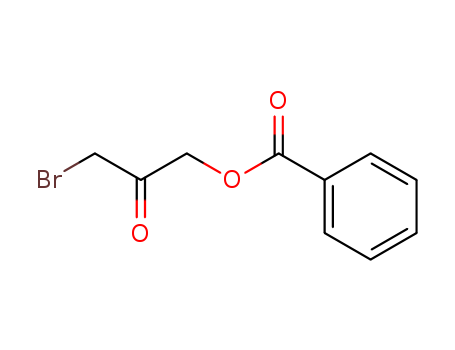
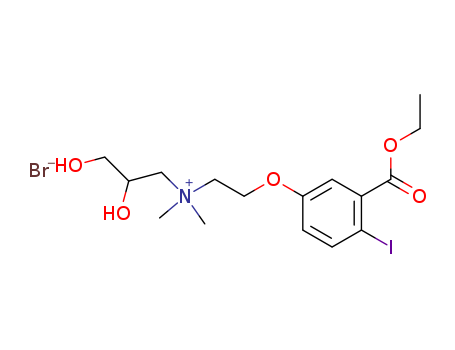
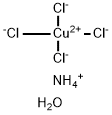
3-BROMO-1,2-PROPANEDIOL literature
HALONIUM ION-INDUCED BIOSYNTHESIS OF CHLORINATED MARINE METABOLITES
Geigert, John,Neidleman, Saul L.,Witt, Susanne K. de,Dalietos, Demetrios J.
, p. 287 - 290 (1984)
Bromoperoxidases do not directly oxidize the chloride ion; nevertheless, in the presence of bromide ions, chloride ions and hydrogen peroxide, bromoperoxidases react with alkenes and alkynes to produce bromochloro-derivatives.This same reaction is catalysed when seawater is the source of chloride and bromide ions.This suggests that bromonium ion-induced biosynthesis of chlorinated metabolites occurs in marine environments.The role of iodonium ions in the biosynthesis of chlorinated metabolites is also discussed.Key Word Index - Coralina sp.; Rhodophyta; biological halogenation; bromoperoxidase; enzymatic bromochlorination; marine chlorination; role of bromonium ions and iodonium ions; seawater.
-
Jones
, p. 697,699 (1973)
-
Bromotrimethylsilane as a selective reagent for the synthesis of bromohydrins
Giomi, Donatella,Salvini, Antonella,Ceccarelli, Jacopo,Brandi, Alberto
, p. 14453 - 14458 (2021/05/19)
Bromotrimethylsilane (TMSBr) is a very efficient reagent in the solvent-free conversion of glycerol into bromohydrins, useful intermediates in the production of fine chemicals. As glycerol is a relevant by-product in biodiesel production, TMSBr has been also tested as a mediator in transesterification in acidic conditions, providing FAME from castor oil in good yields, along with bromohydrins from glycerol. Subsequently the glycerol conversion was optimized and depending on the reaction conditions, glycerol can be selectively converted into α-monobromohydrin (1-MBH) or α,γ-dibromohydrin (1,3-DBH) in very good yields. This journal is
Protective opening of epoxide using pivaloyl halides under catalyst-free conditions
Rao, Chitturi Bhujanga,Rao, Dasireddi Chandra,Venkateswara, Mallem,Venkateswarlu, Yenamandra
supporting information; experimental part, p. 2704 - 2707 (2011/12/05)
An efficient and environmentally benign protocol for protective opening of epoxide (POE) with pivaloyl halides in solvent-free conditions and in aqueous media under catalyst-free conditions has been developed. The green reaction conditions, simple work-up procedures, high yields and broad scope of the reaction illustrate the good synthetic utility of this method. The key advantages of the reaction are regioselectivity and reconvertability of products into their prior epoxides in the presence of mild reaction conditions.
USES AND COMPOSITIONS OF NITRATE ESTERS FOR PROVIDING SEDATION
-
, (2008/06/13)
Use of certain nitrate ester compounds or pharmaceutically acceptable salts thereof in the manufacture of a medicament for treating pain or providing analgesia.
Highly selective epoxidation of olefinic compounds over TS-1 and TS-2 redox molecular sieves using anhydrous urea-hydrogen peroxide as oxidizing agent
Laha,Kumar
, p. 339 - 344 (2007/10/03)
Highly selective epoxidation of different olefinic compounds was carried out using urea-hydrogen peroxide adduct (UHP) as the oxidizing agent in the presence of TS-1 and TS-2 as redox catalysts. A considerable increase in the epoxide selectivity was observed for different unsaturated compounds, such as allylic (allyl alcohol, allyl chloride, allyl bromide, and methylallyl chloride), open-chain, and cyclic (1-hexene and cyclohexene) and aromatic (styrene and allylbenzene) olefinic compounds, when UHP and U + HP (urea and aqueous H2O2 added separately for the in situ formation of UHP) were used as oxidants instead of aqueous H2O2. The controlled release of anhydrous H2O2 from UHP is the main reason for enhanced epoxide selectivity. Direct spectroscopic evidences for the formation of different Ti-superoxo complexes by the solid-solid interaction between TS-1/TS-2 and urea-hydrogen peroxide adduct were obtained from the characteristic continuous absorption band in the UV-vis region (300-500 nm) and the anisotropic EPR spectra for the superoxide radical attached to Ti(IV) centers on TS-1 and TS-2.