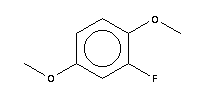
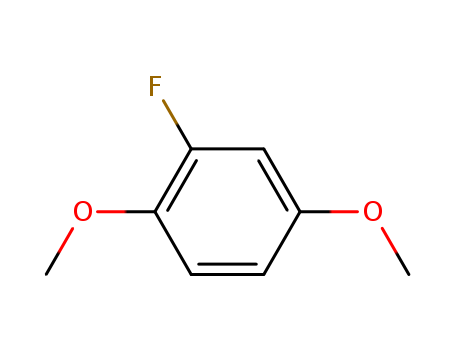
1,4-Dimethoxy-2-fluorobenzene literature
N-arylalkyl-N-heteroarylurea and guandine compounds and methods of treating HIV infection
-
, (2008/06/13)
A method for treating HIV which comprises a compound of the formula STR1 wherein A is STR2 and Zi is O, Se, NRa or C(Ra)2, and Zii is --O or (=O)2 ; wherein R1, R2, R3, and R4 are as defined in the specification.
Phenethylthiazolylthiourea (PETT) compounds as a new class of HIV-1 reverse transcriptase inhibitors. 2. Synthesis and further structure-activity relationship studies of PETT analogs
Cantrell, Amanda S.,Engelhardt, Per,H?gberg, Marita,Jaskunas, S. Richard,Johansson, Nils Gunnar,Jordan, Christopher L.,Kangasmets?, Jussi,Kinnick, Michael D.,Lind, Peter,Morin Jr., John M.,Muesing,Noreén, Rolf,?berg, Bo,Pranc, Paul,Sahlberg, Christer,Ternansky, Robert J.,Vasileff, Robert T.,Vrang, Lotta,West, Sarah J.,Zhang, Hong
, p. 4261 - 4274 (2007/10/03)
Phenylethylthiazolylthiourea (PETT) derivatives have been identified as a new series of nonnucleoside inhibitors of HIV-1 RT. Structure-activity relationship studies of this class of compounds resulted in the identification of N-[2-(2-pyridyl)ethyl]-N'-[2-(5-bromopyridyl)]-thiourea hydrochloride (trovirdine; LY300046.HCl) as a highly potent anti-HIV-1 agent. Trovirdine is currently in phase one clinical trials for potential use in the treatment of AIDS. Extension of these structure-activity relationship studies to identify additional compounds in this series with improved properties is ongoing. A part of this work is described here. Replacement of the two aromatic moleties of the PETT compounds by various substituted or unsubstituted heteroaromatic rings was investigated. In addition, the effects of multiple substitution in the phenyl ring were also studied. The antiviral activities were determined on wild-type and constructed mutants of HIV-1 RT and on wild-type HIV-1 and mutant viruses derived thereof, Ile100 and Cys181, in cell culture assays. Some selected compounds were determined on double- mutant viruses, HIV-1 (Ile100/Asn103) and HIV-1 (Ile100/Cys181). A number of highly potent analogs were synthesized. These compounds displayed IC50's against wild-type RT between 0.6 and 5 nM. In cell culture, these agents inhibited wild-type HIV-1 with ED50's between I and 5 nM in MT-4 cells. In addition, these derivatives inhibited mutant HIV-1 RT (Ile 100) with IC50's between 20 and 50 nM and mutant HIV-1 RT (Cys 181) with IC50's between 4 and 10 nM, and in cell culture they inhibited mutant HIV-1 (Ile100) with ED50's between 9 and 100 nM and mutant HIV-1 (Cys181) with ED50's between 3 and 20 nM.
Selective, Electrophilic Fluorinations Using N-Fluoro-o-benzenedisulfonimide
Davis, Franklin A.,Han, Wei,Murphy, Christopher K.
, p. 4730 - 4737 (2007/10/02)
The synthesis of N-fluoro-o-benzenedisulfonimide (NFOBS, 2) and its use as an "electrophilic" fluorinating reagent with nucleophilic substrates is described and compared with that of N-fluorobenzenesulfonimide (NFSi, 3).NFOBS (2) is prepared in three steps in 81percent overall yield from commercially available o-benzenedisulfonic acid (4) and involves treatment of o-benzenedisulfonimide (6) with dilute fluorine (10percent F2/N2).Reaction of 2 with metal enolates, silyl enol ethers, and 1,3-dicarbonyl compounds affords the corresponding α-fluoro compounds in yields up to 95percent, with good control of mono- and difluorination.Fluorination of ortho-metalated aromatic compounds was achieved in modest to good yields (10-80percent).While the reactivities of 2 and 3 are similar, better yields were observed with the former reagent in the fluorination of metal enolates, Grignard and lithium reagents, while 3 gave better results with the ortho-lithiated aromatic substrates.The available evidence suggests an SN2-type mechanism for the fluorination of nucleophilic substrates by these reagents.
Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane
Glennon,Young,Benington,Morin
, p. 1163 - 1168 (2007/10/02)
The serotonin (5-HT) receptor affinities and behavioral (discriminative stimulus) properties of a series of 4-substituted derivatives of 1-(2,5-dimethoxyphenyl)-2-aminopropanes (2,5-DMA) were investigated. The substituents at the 4-position included H, OMe, OEt, Me, Et, F, Br, I, and NO2. Substituent lipophilicities (π values) of these functionalities appear to have a minimal effect on either 5-HT receptor affinity or behavioral activity. Those derivatives previously found to be most potent in human studies possess significant affinity for 5-HT receptors. Furthermore, when rats trained to discriminate (±)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) from saline were used, generalization was found to occur upon administration of the 4-substituted 2,5-DMA derivatives. Because a direct relationship exists between the ED50 values obtained from these discrimination studies and human hallucinogenic potencies, the discriminative stimulus paradigm, with DOM as a training drug, appears to be a useful tool for comparing the quantitative and qualitative (DOM-like) effects produced by certain hallucinogenic agents.