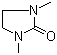
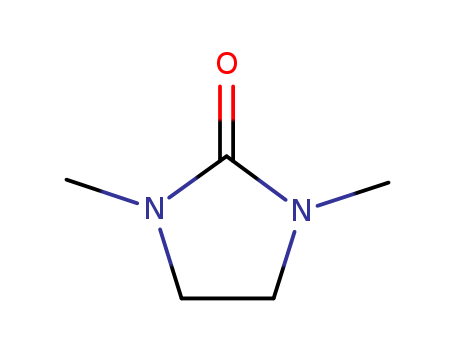
1,3-Dimethyl-2-imidazolidinone literature
-
Yoder,Zuckerman
, p. 694 (1966)
-
Reaction of 3,4-Di-t-butylthiophene 1-oxide with 2-methylene-1,3-dimethylimidazolidine: Methylene transfer and [4+4] dimerization
Nakayama, Juzo,Takayama, Jun,Sugihara, Yoshiaki,Ishii, Akihiko
, p. 758 - 759 (2001)
The reaction of 3,4-di-t-butylthiophene 1-oxide (1) with 2-methylene-1,3-dimethylimidazolidine (2) gave 4,4a-di(t-butyl)-1a,4a-dihydro-1H-cyclopropa[b]thiophene and 1,3-dimethyl-2-imidazolidinone through a methylene transfer from 2 to 1, in addition to a [4+4] cyclodimerization product of 1.
A DIPOLE MOMENT STUDY OF N-METHYL AND N,N'-DIMETHYL-IMIDAZOLIDIN-2-ONES, IMIDAZOLIDINE-2-THIONES AND -2-SELENONES
Lumbroso, H.,Liegeois, Ch.,Devillanova, F. A.,Verani, G.
, p. 239 - 252 (1981)
The electric dipole moments in benzene and dioxan of potentially tautomerizable N-methylimidazolidin-2-one, N-methylimidazolidine-2-thione and -2-selenone clearly support the lactam structure for these compounds.The fact that their dipole moments in dioxan are markedly greater than those in benzene is explained by a higher (HN-C=Y) mesomeric moment in the hydrogen-bonded solute...dioxan complexes.Analysis of the dipole moments in benzene of N,N'-dimethylimidazolidin-2-one, N,N'dimethylimidazolidine-2-thione and -2-selenone shows that the mesomeric moment (due to contribution of +N=C-Y- zwitterionic valence structures) gradually increases on going from Y=O to Y=S, and Y=Se.Finally, preferred conformations, from their dipole moments in benzene, are suggested for tetramethylurea and tetramethylthiourea.
Mesoporous silica-catalysed continuous chemical fixation of CO2 with N,N′-dimethylethylenediamine in supercritical CO2: The efficient synthesis of 1,3-dimethyl-2-imidazolidinone
Seki, Tsunetake,Kokubo, Yoshiaki,Ichikawa, Shinichiro,Suzuki, Tomoyuki,Kayaki, Yoshihito,Ikariya, Takao
, p. 349 - 351 (2009)
MCM-41- and HMS-type mesoporous silicas were found to be efficient catalysts for the continuous chemical fixation of CO2 into 1,3-dimethyl-2-imidazolidinone with N,N′-dimethylethylenediamine in supercritical CO2. The Royal Society of Chemistry.
Optochemical Control of Bacterial Gene Expression: Novel Photocaged Compounds for Different Promoter Systems
Bier, Claus,Binder, Dennis,Bitzenhofer, Nora Lisa,Drepper, Thomas,Haase, Mona,Hilgers, Fabienne,Hogenkamp, Fabian,Jaeger, Karl-Erich,Ophoven, Vera,Pietruszka, J?rg
, (2021/12/06)
Photocaged compounds are applied for implementing precise, optochemical control of gene expression in bacteria. To broaden the scope of UV-light-responsive inducer molecules, six photocaged carbohydrates were synthesized and photochemically characterized, with the absorption exhibiting a red-shift. Their differing linkage through ether, carbonate, and carbamate bonds revealed that carbonate and carbamate bonds are convenient. Subsequently, those compounds were successfully applied in vivo for controlling gene expression in E. coli via blue light illumination. Furthermore, benzoate-based expression systems were subjected to light control by establishing a novel photocaged salicylic acid derivative. Besides its synthesis and in vitro characterization, we demonstrate the challenging choice of a suitable promoter system for light-controlled gene expression in E. coli. We illustrate various bottlenecks during both photocaged inducer synthesis and in vivo application and possibilities to overcome them. These findings pave the way towards novel caged inducer-dependent systems for wavelength-selective gene expression.
SBA-15 Supported Dendritic ILs as a Green Catalysts for Synthesis of 2-Imidazolidinone from Ethylenediamine and Carbon Dioxide
Liu, Jinghan,Ma, Jianjun,Miao, Penghua,Min, Qingwang,Qi, Meijuan,Shamsa, Farzaneh
, (2021/07/26)
In this work, a simple and facile approach is conducted for preparing many new SBA-15 supported dendritic imidazolium ILs heterogeneous catalysts SBA-15/IL(1–3) having high ionic density from SBA-15. SBA-15/IL(3) as a green heterogeneous catalyst can be used for synthesis of 2-imidazolidinone from ethylenediamine and carbon dioxide and considering solvent-free condition. SBA-15/IL(3) showed to have the highest catalytic activity besides a positive dendritic influence on the yields of the synthesis of 2-imidazolidinone in the presence of CO2 is seen because of existing the high-density peripheral zwitterionic ionic liquid functional groups on the biobased SBA-15/IL(3) catalyst surfaces. Graphical Abstract: [Figure not available: see fulltext.]
Reaction of Nitroxyl (HNO) with Hydrogen Sulfide and Hydropersulfides
Zarenkiewicz, Jessica,Khodade, Vinayak S.,Toscano, John P.
, p. 868 - 877 (2021/01/14)
Nitroxyl (HNO) has gained a considerable amount of attention because of its promising pharmacological effects. The biochemical mechanisms of HNO activity are associated with the modification of regulatory thiol proteins. Recently, several studies have suggested that hydropersulfides (RSSH), presumed signaling products of hydrogen sulfide (H2S)-mediated thiol (RSH) modification, are additional potential targets of HNO. However, the interaction of HNO with reactive sulfur species beyond thiols remains relatively unexplored. Herein, we present characterization of HNO reactivity with H2S and RSSH. The reaction of H2S with HNO leads to the formation of hydrogen polysulfides and sulfur (S8), suggesting a potential role in sulfane sulfur homeostasis. Furthermore, we show that hydropersulfides are more efficient traps for HNO than their thiol counterparts. The reaction of HNO with RSSH at varied stoichiometries has been examined with the observed production of various dialkylpolysulfides (RSSnSR) and other nitrogen-containing dialkylpolysulfide species (RSS-NH-SnR). We do not observe evidence of sulfenylsulfinamide (RS-S(O)-NH2) formation, a pathway expected by analogy with the known reactivity of HNO with thiol.
Alkylamine-Substituted Perthiocarbamates: Dual Precursors to Hydropersulfide and Carbonyl Sulfide with Cardioprotective Actions
Khodade, Vinayak S.,Pharoah, Blaze M.,Paolocci, Nazareno,Toscano, John P.
, p. 4309 - 4316 (2020/03/05)
The recent discovery of hydropersulfides (RSSH) in mammalian systems suggests their potential roles in cell signaling. However, the exploration of RSSH biological significance is challenging due to their instability under physiological conditions. Herein, we report the preparation, RSSH-releasing properties, and cytoprotective nature of alkylamine-substituted perthiocarbamates. Triggered by a base-sensitive, self-immolative moiety, these precursors show efficient RSSH release and also demonstrate the ability to generate carbonyl sulfide (COS) in the presence of thiols. Using this dually reactive alkylamine-substituted perthiocarbamate platform, the generation of both RSSH and COS is tunable with respect to half-life, pH, and availability of thiols. Importantly, these precursors exhibit cytoprotective effects against hydrogen peroxide-mediated toxicity in H9c2 cells and cardioprotective effects against myocardial ischemic/reperfusion injury, indicating their potential application as new RSSH- and/or COS-releasing therapeutics.