1,1-Cyclohexanediacetic acid mono amide literature
Preparation method and application of 1-1 - cyclohexane - diacetic acid monoamide
-
Paragraph 0141-0144, (2021/09/29)
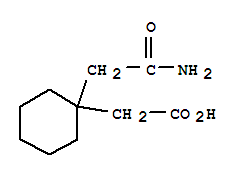
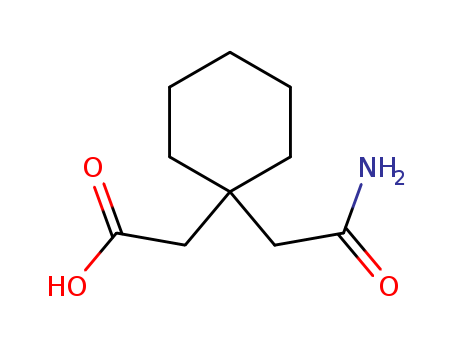
The invention discloses a preparation method of 1-1 - cyclohexane - diacetic acid monoamide (CDMA), which comprises an amidation reaction of 1, 1 - cyclohexanedicarboxylic acid anhydride and 1 and 1 - cyclohexane - diacetic acid monoamide ammonium salt in a non-polar solvent of non-benzene to obtain 1, 1 - cyclohexane - diacetic acid monoamide (CDMA). Alternatively, in the non-polar solvent of non-benzene, 1, 1 - cyclohexanedicarboxylic anhydride is reacted with ammonia to heat reflux to give 3, 3 - cyclopentanediimides. 3,3 - Cycloglutarimide is obtained 1 by untying a base water, 1 - cyclohexane - diacetic acid monoamide (CDMA) wherein, with 1, 1 - cyclohexanedicarboxylic acid as a starting material, dehydration is carried out under acid catalysis to give 1, 1 - cyclohexanedicarboxylic acid anhydride.
Preparation method of gabapentin intermediate
-
Paragraph 0076; 0082-0085; 0086-0091-0093; 0094; 0099-0101, (2021/04/03)
The invention provides a preparation method of a gabapentin intermediate, and relates to the field of chemical organic synthesis. The preparation method comprises the following steps: taking cyanoacetic acid and cyclohexanone as raw materials, carrying out condensation, hydrolysis and decarboxylation reactions to obtain imide in an intermediate body, and further carrying out alkaline hydrolysis toobtain the intermediate cyclohexyl diacetate monoamide. The preparation method of the gabapentin intermediate provided by the invention has the advantages of readily available raw materials, mild reaction conditions, high safety factor, strong operability, simple process, easiness in industrialization, high product purity and stable quality.
Preparation method of glutaryl imide derivative
-
Paragraph 0037; 0039-0040; 0044; 0046-0047; 0051; 0053-0054, (2021/03/31)
The invention discloses a preparation method of a glutaryl imide derivative, which comprises the following steps: in a negative pressure state, dropwise adding acetic anhydride into molten 1, 1-cyclohexyl diacetic acid, and reacting to obtain 1, 1-cyclohexyl diacetic anhydride; adding ammonia water into an ammoniation kettle, dropwise adding 1, 1-cyclohexanediacetic anhydride to carry out ammoniation reaction, and adding hydrochloric acid to adjust the pH value, so as to obtain precipitated crystals, namely pentane valeric acid; adding pentane valeric acid, a toluene solvent and glacial aceticacid into the reaction kettle, heating, stirring, reacting, cooling, and carrying out suction filtration to obtain a filter cake; and adding the filter cake into ammonia water for soaking and stirring, carrying out suction filtration again, leaching with deionized water, and drying to obtain glutaryl imide. According to the preparation method of a glutaryl imide derivative, acetic anhydride and 1, 1-cyclohexyldiacetic acid are used as raw materials, so that the reaction efficiency is effectively improved, the product yield is increased, the production cost of the product is reduced, and producing benefits are improved.
Process for the preparation of gabapentin
-
Paragraph 6; 7, (2019/09/17)
The present invention relates to an improved process for the preparation of Gabapentin. The process also relates to a new process for the preparation of 1, 1-cyclohexane diaceitic acid monoamide (CDMA), which is a key intermediate for the preparation of Gabapentin.