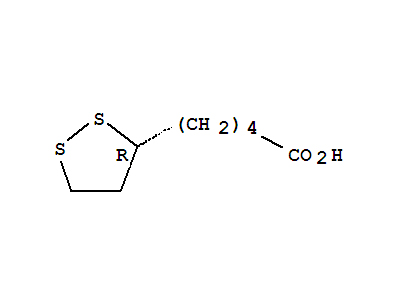
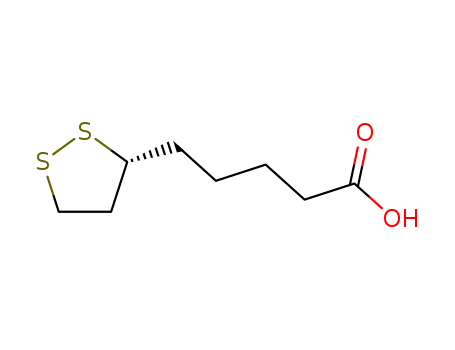
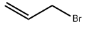
(R)-(+)-1,2-Dithiolane-3-pentanoic acid literature
Enantioselective Synthesis of R-(+)-α-Lipoic Acid
Page, Philip C. Bulman,Rayner, Christopher M.,Sutherland, Ian O.
, p. 1408 - 1409 (1986)
The title compound has been synthesised in an enantioselective manner from achiral precursors using the Sharpless asymmetric epoxidation as the key step in the reaction sequence.
ASYMMETRIC SYNTHESIS VIA ACETAL TEMPLATES. 12. HIGHLY DIASTEREOSELECTIVE COUPLING REACTIONS WITH A KETENE ACETAL. AN EFFICIENT, ASYMMETRIC SYNTHESIS OF R-(+)-α-LIPOIC ACID.
Elliott, John D.,Steele, John,Johnson, William S.
, p. 2535 - 2538 (1985)
TiCl4 catalyzes the essentially quantitative coupling of chiral acetals 1 with 1-t-butoxy-1-t-butyldimethylsilyloxyethene 2 to generate β-alkoxycarboxylates in wich the new asymmetric center is formed with excellent diastereoselection. β-Hydrocarboxylic acids of high ee result from removal of the chiral auxiliary.The procedure has been appllied to the synthesis of R-(+)-α-lipoic acid 10.
Coevolution of the Activity and Thermostability of an ?-Keto Ester Reductase for Better Synthesis of an (R)-α-Lipoic Acid Precursor
Chen, Qi,Xu, Jian-He,Xu, Yao,Zhang, Zhi-Jun,Zheng, Gao-Wei
, (2019)
In this work, we have identified a significantly improved variant (S131Y/Q252I) of the natural ?-keto ester reductase CpAR2 from Candida parapsilosis for efficiently manufacturing (R)-8-chloro-6-hydroxyoctanoic acid [(R)-ECHO] through co-evolution of activity and thermostability. The activity of the variant CpAR2S131Y/Q252I towards the ?-keto ester ethyl 8-chloro-6-oxooctanoate was improved to 214 U mg?1—from 120 U mg?1 in the case of the wild-type enzyme (CpAR2WT)—and the half-deactivating temperature (T50, for 15 min incubation) was simultaneously increased by 2.3 °C in relation to that of CpAR2WT. Consequently, only 2 g L?1 of lyophilized E. coli cells harboring CpAR2S131Y/Q252I and a glucose dehydrogenase (GDH) were required in order to achieve productivity similar to that obtained in our previous work, under optimized reaction conditions (530 g L?1 d?1). This result demonstrated a more economical and efficient process for the production of the key (R)-α-lipoic acid intermediate ethyl 8-chloro-6-oxooctanoate.
Stereocontrolled reactions induced by a thermolabile group. Synthesis of optically active 1,3-diols
Bloch, Robert,Bortolussi, Michel,Girard, Christian,Seck, Matar
, p. 453 - 462 (1992)
Wittig Horner-Michael reactions of phosphonates with optically active lactol 1 lead preferentially to one diastereoisomer. 2. Force field calculations conducted on one pair of diastereoisomers 2c and 2′c predict that these isomers must exist in different conformations of similar energies. 1H NMR data are in good agreement with these predictions. The dihydrofurans obtained by retro Diels-Alder reactions of 2 are easily transformed into optically pure 1,3-diols, precursors of R-(+)-α-lipoic acid and (-)-(1R,3R,5s)-1,3-dimethyl-2,9-dioxabicylco [3.3.1]nonane.
Microwave-assisted resolution of α-lipoic acid catalyzed by an ionic liquid co-lyophilized lipase
Liu, Ning,Wang, Lei,Wang, Zhi,Jiang, Liyan,Wu, Zhuofu,Yue, Hong,Xie, Xiaona
, p. 9949 - 9960 (2015)
The combination of the ionic liquid co-lyophilized lipase and microwave irradiation was used to improve enzyme performance in enantioselective esterification of α-lipoic acid. Effects of various reaction conditions on enzyme activity and enantioselectivity were investigated. Under optimal condition, the highest enantioselectivity (E = 41.2) was observed with a high enzyme activity (178.1 μmol/h/mg) when using the ionic liquid co-lyophilized lipase with microwave assistance. Furthermore, the ionic liquid co-lyophilized lipase exhibited excellent reusability under low power microwave.
Preparation method of D-lipoic acid
-
Paragraph 0040-0070, (2021/02/24)
The invention relates to a preparation method of d-lipoic acid. The preparation method comprises the following steps: carrying out free reaction on a compound shown as a formula I under the action ofcitric acid, washing with water, concentrating, and eluting to obtain the d-lipoic acid. According to the scheme for preparing the d-lipoic acid, the generation of polymer related substances in the preparation process of the d-lipoic acid can be remarkably reduced.
Preparation method of R-lipoic acid
-
, (2021/07/31)
The invention discloses a preparation method of R-lipoic acid, and belongs to the technical field of pharmaceutical chemistry synthesis. The method comprises the following steps: preparing an intermediate-1; preparing an intermediate-2; preparing an intermediate-3; preparing an intermediate-4; preparing an intermediate-5; preparing an intermediate-6; preparing an intermediate-7; and preparing a finished product, and performing hydrolysis reaction on the obtained intermediate-7 under an alkaline condition to obtain R-lipoic acid. The method has the advantages of mild process conditions, high optical purity of chiral intermediates and final products, and facilitation of quality control and improvement of bulk drugs of the final products; and reagent raw materials used in the process route are easy to obtain, the technical scheme is reasonable and environment-friendly, and the method can meet the use requirements through mass production and is suitable for industrial large-scale production.
Synthesis method R -lipoic acid
-
, (2021/09/26)
A synthesis method of R -lipoic acid comprises N - (5 -bromopentyl) phthalimide and zinc powder. Lithium chloride, trimethylchlorosilane, 1,2 - dibromoethane and tetrahydrofuran are mixed, reacted to form a zinc bromide intermediate, and 3 - {[2 - (trimethylsilyl) ethoxy] methoxy} propionyl chloride is then reacted with a zinc bromide intermediate. The obtained intermediate -1 is subjected to a hydrazine decomposition reaction. The obtained intermediate -2 is subjected to a catalytic oxidation reaction. The obtained intermediate -3 is subjected to an esterification reaction. The nearly smooth Candida tropicalis is introduced into a fermentation medium for amplification culture, and the obtained resting cells are suspended in a buffer aqueous solution and the obtained intermediate -4 is added for enzyme-catalyzed chiral reduction reaction. The resulting intermediate -5 is subjected to deprotection reaction. The resulting intermediate -6 is subjected to a chlorination reaction. The resulting intermediate -7 was subjected to a cyclization reaction. The obtained intermediate -8 was subjected to a hydrolysis reaction to obtain a finished product.
Preparation method of R-lipoic acid tromethamine salt
-
Paragraph 0028; 0036; 0038; 0045; 0047; 0054, (2021/05/12)
The invention relates to a preparation method of R-lipoic acid trometamol salt, which comprises the following steps: introducing candida parapsilosis into a fermentation culture medium for multiplication culture, and conducting centrifugal separation; suspending obtained resting cells in a buffer aqueous solution, adding ethyl 6-carbonyl-8-chlorocaprylate, glucose dehydrogenase, glucose and nicotinamide adenine dinucleotide, and carrying out a chiral reduction reaction; mixing the obtained ethyl S-6-hydroxy-8-chlorocaprylate with a chlorination reagent, a catalyst and a solvent, and carrying out a chlorination reaction; adding the obtained ethyl R-6,8-dichlorocaprylate into a system of sulfur, sodium sulfide, a phase transfer catalyst and water, and carrying out cyclization reaction; carrying out hydrolysis reaction on the obtained R-lipoic acid ethyl ester under an alkaline condition; and suspending the obtained R-lipoic acid and trometamol in an alcohol solvent, conducting heating and dissolving, and carrying out salt forming reaction to obtain a finished product. The method has the advantages of mild process conditions, low cost, high product purity, high yield and high optical purity.