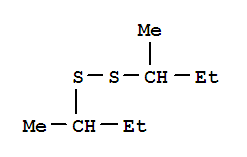
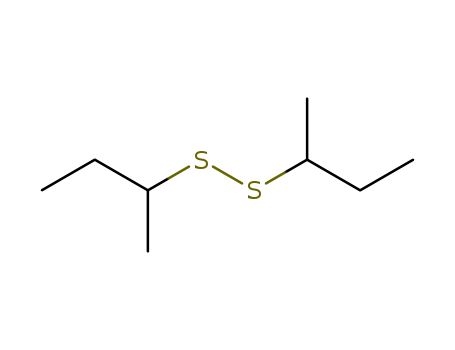
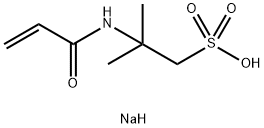
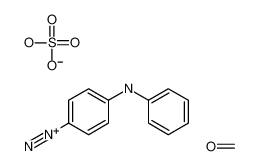
sec-Butyl disulfide literature
Synthesis of sec-butyl disulfide by phase transfer catalysis
Liu, Xinqi,Wang, Jia,Zhao, Songfang,Hu, Guoqin
, p. 3208 - 3210 (2015)
Sec-Butyl disulfide was synthesized from sulfur?sodium hydroxide and sec-butyl chloride using tetrabutyl ammonium bromide as the phase transfer catalyst. The optimal experiment condition obtained by orthogonal design and the average product yield was 84.95 %. The samples synthesized under optical conditions were characterized by infrared spectrometry?gas chromatography-mass spectrometer.
The synthesis of symmetrical disulfides by reacting organic halides with Na2S2O3·5H2O in DMSO
Abbasi, Mohammad,Mohammadizadeh, Mohammad Reza,Saeedi, Narges
supporting information, p. 89 - 92 (2016/01/12)
A one-pot, and scalable method to prepare symmetric disulfides from their corresponding primary, secondary, allylic, and benzylic halides has been developed. In this method, a disulfide is synthesized by reacting an alkyl halide with Na2S2O3·5H2O at 60-70 °C in DMSO.
Graphene Oxide-Assisted One-Pot and Odorless Synthesis of Symmetrical Disulfides Using Primary and Secondary Alkyl Halides (Tosylates) and Thiourea as Sulfur Source Reagent
Khalili, Dariush
, p. 1727 - 1734 (2015/12/12)
Graphene oxide is described as a heterogeneous oxidant for the synthesis of symmetrical disulfides through the in situ generation of thiols from primary and secondary alkyl halides (tosylates) and thiourea in wet acetonitrile. A variety of alkyl halides and alkyl tosylates can be converted to corresponding disulfides in good to excellent yields. This strategy is free of foul-smelling thiols.
4,4'-Azopyridine as an easily prepared and recyclable oxidant for synthesis of symmetrical disulfides from thiols or alkyl halides(tosylates)/thiourea
Khalili, Dariush,Iranpoor, Nasser,Firouzabadi, Habib
, p. 544 - 555 (2015/10/19)
Heterocyclic azo compounds, prepared from corresponding amines in one step, are used as effective oxidants for the conversion of thiols into symmetrical disulfides in high yields. Among the studied azo compounds, 4,4'-azopyridine was found to be very efficient for the odorless conversion of alkyl halides into disulfides in the presence of thiourea. An attractive feature of this azo compound is that its obtained solid side product hydrazine is easily separated by filtration and can be recycled to its azo compound for further use.