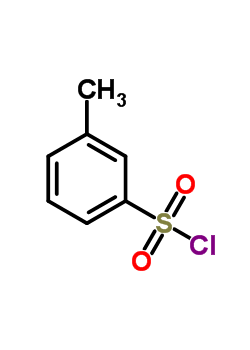
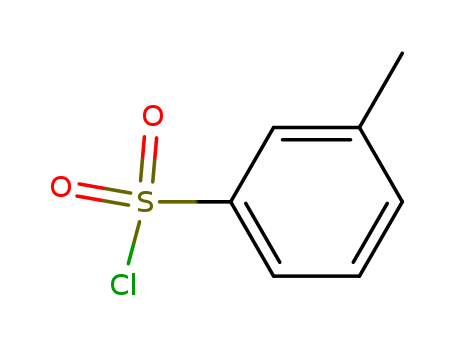
m-Toluenesulfonyl chloride literature
Aminoxidation of Arenethiols to N-Chloro-N-sulfonyl Sulfinamides
Yang, Zhanhui,Xu, Wei,Wu, Qiuyue,Xu, Jiaxi
, p. 3051 - 3057 (2016/04/26)
A simple and efficient method to synthesize N-chloro-N-sulfonylsulfinamides by the direct aminoxidation of arenethiols under aqueous and mild conditions is disclosed, geminally installing the oxo and amino groups on the sulfur atom of arenethiols. The products have been primarily developed as sulfinylation reagents to convert Grignard reagents into sulfoxides, and as amination reagents to convert secondary amines into hydrazine derivatives.
New process for the production of arensulfonyl chloride from arensulfonic acid
-
Paragraph 0028; 0029, (2016/12/26)
The present invention refers to degradation of a fine chemicals functionalised in a synthetic process of intermediates to conversion of an important role new manufacturing method relates to a novel process of arylsulfonyl, arylsulfonyl chloride hour draw from previous techniques are nick biting opinion gun method for producing [...] the users determine a structure of basic presents at 70 degree or more time and an elongated reactant hot reaction of undesirable and uniform reaction conditions to improve power efficiency nick biting opinion gun a dilute bis (trichloromethyl) polycarbonate and a catalytic amount of potassium and phosphoric acid incorporated tetrahedral amorphous [...] triethylamine and tree hereinafter also 25 reacting under a condition of gently arylsulfonyl chloride characterized by high yield in the dye by providing new method, pesticide, medicine, as well as related industrial electronic materials of the field will hole is compressed by the reciprocal movement.
DABCO-bis (sulfur dioxide), DABSO, as a convenient source of sulfur dioxide for organic synthesis: Utility in sulfonamide and sulfamide preparation
Woolven, Holly,Gonzalez-Rodriguez, Carlos,Marco, Isabel,Thompson, Amber L.,Willis, Michael C.
supporting information; experimental part, p. 4876 - 4878 (2011/12/05)
The charge-transfer complex generated from the combination of DABCO and sulfur dioxide, DABSO, is a bench-stable colorless solid suitable for use in organic synthesis as a replacement for gaseous sulfur dioxide. The complex can be combined with Grignard reagents to form sulfinates, which can then be converted in situ to a series of sulfonamides. Alternatively, reaction with anilines and iodine leads to the formation of a series of sulfamides. Cheletropic addition between DABSO and 2,3-dimethylbutadiene provides the corresponding sulfolene.
Orally active aminopyridines as inhibitors of tetrameric fructose-1,6-bisphosphatase
Hebeisen, Paul,Haap, Wolfgang,Kuhn, Bernd,Mohr, Peter,Wessel, Hans Peter,Zutter, Ulrich,Kirchner, Stephan,Ruf, Armin,Benz, J?rg,Joseph, Catherine,Alvarez-Sánchez, Rubén,Gubler, Marcel,Schott, Brigitte,Benardeau, Agnes,Tozzo, Effie,Kitas, Eric
scheme or table, p. 3237 - 3242 (2011/07/07)
A novel sulfonylureido pyridine series exemplified by compound 19 yielded potent inhibitors of FBPase showing significant glucose reduction and modest glycogen lowering in the acute db/db mouse model for Type-2 diabetes. Our inhibitors occupy the allosteric binding site and also extend into the dyad interface region of tetrameric FBPase.