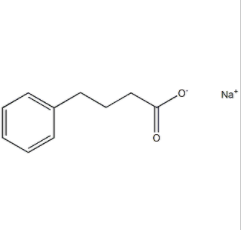
Sodium 4-phenylbutyrate literature
Intermolecular Reductive Heck Reaction of Unactivated Aliphatic Alkenes with Organohalides
Zheng, Kewang,Xiao, Guanlin,Guo, Tao,Ding, Yalan,Wang, Chengdong,Loh, Teck-Peng,Wu, Xiaojin
, p. 694 - 699 (2020/01/31)
A general intermolecular reductive Heck reaction of organohalides with both terminal and internal unactivated aliphatic alkenes has been first realized in high yield with complete anti-Markovnikov selectivity. The challenging vinyl bromides, aryl chlorides, and polysubstituted internal alkenes were first applied. More than 100 remote carbofunctionalized alkyl carboxylic acid derivatives were rapidly synthesized from easily accessible starting materials. The synthesis of drug molecules has further demonstrated the wide synthetic utility of this scalable strategy. Preliminary mechanistic studies are consistent with the proposed catalytic cycle.
Preparation method of sodium phenylbutyrate
-
Paragraph 0014; 0028-0032, (2019/10/22)
The invention discloses a preparation method of sodium phenylbutyrate. The preparation method comprises the steps that in the inert atmosphere, materials and anhydrous 2,3-butanediol are added into areaction vessel, and mixing is conducted uniformly, wherein the related materials include allyl palladium (II) chloride dimer, 2-(dicyclohexylphosphino)-1-phenyl-1H-pyrrole, N-(8-aminoquinoline)butyl-3-enamide, lithium acetate, bromobenzene, cyanoacetic acid and water; the reaction vessel is placed in an oil bath at 125-135 DEG, a vigorous stirring reaction is conducted for 12 hours, a reaction product is purified through a silica gel column, and a compound with a guiding group is obtained; the compound is added into an ethanol solvent containing sodium hydroxide, the mixture is heated to 130-140 DEG C, a reflux reaction is conducted for 12 hours, the reaction product is subjected to vacuum distillation to remove the solvent, extraction is conducted, a water layer is collected, vacuum distillation is conducted to remove water, and a sodium phenylbutyrate preparation is obtained. The method has the advantages of being high in reaction site selectivity and yield, mild in reaction condition and simple in reaction and aftertreatment purification process.
Preparation method for sodium phenylbutyrate
-
Paragraph 0084; 0089, (2017/06/29)
The invention discloses a preparation method for sodium phenylbutyrate. The method comprises the following steps: (1) purification of 4-phenylbutyric acid: 1) under the catalysis of a catalyst, reacting industrial grade phenylbutyric acid in alcoholic solvents, and treating a reacted system to obtain the 4-phenylbutyric acid; 2) in the existence of an alkali catalyst or an acidic catalyst, performing hydrolysis reaction on the 4-phenylbutyric acid in a solvent to obtained purified phenylbutyric acid, namely, the industrial grade phenylbutyric acid is purified; (2) preparation of the sodium phenylbutyrate: enabling the phenylbutyric acid which is purified by the step 1) to react with a sodium reagent to obtain the sodium phenylbutyrate. According to the preparation method disclosed by the invention, alcohols (methyl alcohol and ethyl alcohol) serve as reaction solvents, so that the preparation method is more environmentally -friendly and green compared with a synthesis method in the prior art; the preparation of high-purity methyl alcohol is realized through a three-step conventional reaction; the purity of the sodium phenylbutyrate prepared by the preparation method reaches 99.5 percent or above, and single impurities are controlled to be within 0.1 percent.
Use of 4-phenylbutyric acid and/or salts thereof for enhancing stress tolerance in plants
-
Page/Page column 3, (2012/04/05)
The invention relates to the use of 4-phenylbutyric acid and/or salts thereof, of the formula (I) for enhancing stress tolerance in plants to abiotic stress, preferably drought stress, and to the associated enhancement in plant growth and/or increase in plant yield.