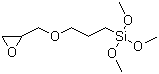
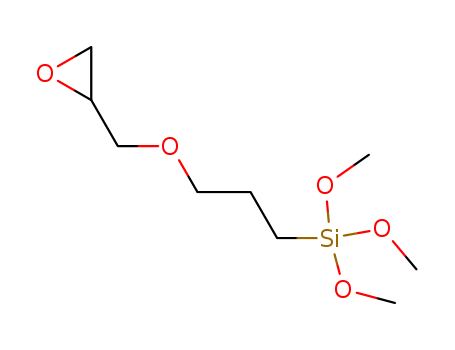
3-Glycidoxypropyltrimethoxysilane literature
Process for preparing 3-glycidyloxypropyltrialkoxysilanes
-
Paragraph 0139-0142; 0144-0153, (2019/02/24)
A process can prepare a 3-glycidyloxypropylalkoxysilane of formula (I), (R′)O—(CH2)3—Si(OR)3 (I), where R groups are independently a methyl or ethyl group and R′ represents an H2C(O)CHCH2— group. The process includes reacting (i) a functionalized alkene of formula (II), (R′)O—C3H5 (II), where R′ represents an H2C(O)CHCH2— group, with (ii) at least one hydroalkoxysilane of formula (III), HSi(OR)3 (III), where R groups are independently a methyl or ethyl group. The reacting takes place in the presence of (iii) a Karstedt catalyst or a catalyst having hexachloroplatinic acid as a homogeneous catalyst, and (iv) 2-ethylhexanoic acid, isononanoic acid, or both. The process further includes obtaining a product of the reacting.
An Easily Accessed Nickel Nanoparticle Catalyst for Alkene Hydrosilylation with Tertiary Silanes
Buslov, Ivan,Song, Fang,Hu, Xile
supporting information, p. 12295 - 12299 (2016/10/13)
The first efficient and non-precious nanoparticle catalyst for alkene hydrosilylation with commercially relevant tertiary silanes has been developed. The nickel nanoparticle catalyst was prepared in situ from a simple nickel alkoxide precatalyst Ni(OtBu)2?x KCl. The catalyst exhibits high activity for anti-Markovnikov hydrosilylation of unactivated terminal alkenes and isomerizing hydrosilylation of internal alkenes. The catalyst can be applied to synthesize a single terminal alkyl silane from a mixture of internal and terminal alkene isomers, and to remotely functionalize an internal alkene derived from a fatty acid.
Method for preparing organic silicon by passage reaction device
-
Paragraph 0049, (2016/10/17)
The invention provides a method for preparing organic silicon by a passage reaction device. Under the condition of main catalysts Z, hydrogen-containing silane X and an unsaturated compound Y are introduced into the passage reaction device; hydrosilylation reaction is performed to prepare the organic silicon, wherein the hydrogen-containing silane X has the structure being HSiRR'Cl, in the formula, R and R' are independently C1 to C16 alkyl or alkoxy; a=1, 2 or 3; b, c and d are respectively and independently 0, 1, 2 or 3; the unsaturated compound Y is a monoene compound or single-alkyne compound; the main catalysts Z are one or several mixed ones of single-component complexes or multi-component complexes of Pt, Pd, Rh, Ru, Cu, Ag, Au or Ir; the passage surface in which reaction flow contacts is subjected to inactivation treatment by an activating agent Z. The problems of long reaction period, poor stability and the like of large-sized reaction equipment are solved; the problem that mixing, pre-reaction and afterreaction are separated and are performed in multi-unit equipment is solved.
COSMETIC TREATMENT METHOD COMPRISING THE APPLICATION OF A COATING BASED ON AN AEROGEL COMPOSITION OF LOW BULK DENSITY
-
Paragraph 0069, (2014/02/15)
The present invention relates to a cosmetic treatment method comprising the formation of a coating on keratin fibres characterized in that it comprises: 1) the preparation of an aerogel precursor composition comprising:—at least one organic solvent chosen from acetone, C1-C4 alcohols, C1-C6 alkanes, C1-C4 ethers, which may or may not be perfluorinated, and mixtures thereof and at least one precursor compound that contains:—at least one atom chosen from silicon, titanium, aluminium and zirconium,—at least one hydroxyl or alkoxy function directly attached to the atom chosen from silicon, titanium, aluminium and zirconium by an oxygen atom, and,—optionally an organic group directly attached to the atom chosen from silicon, titanium, aluminium and zirconium by a carbon atom, 2) the removal of the solvent or solvents resulting in the formation of an aerogel composition having a bulk density less than or equal to 0.35 g/cm3, 3) the application to the keratin fibres of the aerogel composition resulting from step 2) or of the aerogel precursor composition resulting from step 1). Advantageously, the molar ratio between the precursor compounds and the solvent is at most 1/20.