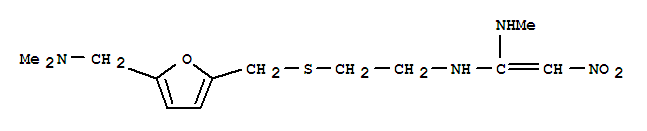Ranitidine hydrochloride literature
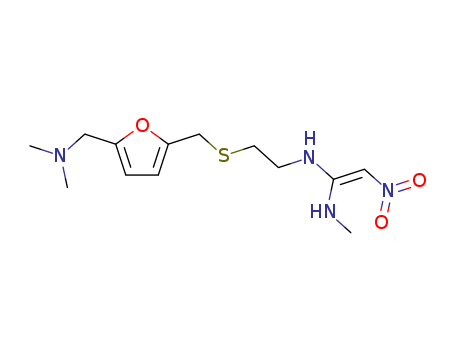
An improved synthesis of the antiulcer drug ranitidine from N-[2-[[[5- (hydroxymethyl)-2-furanyl]-methyl]-thio]-ethyl]-N'-methyl-2-nitro-1,1- ethenediamine
Aasen, Arne Jorgen,Skramstad, Jan
, p. 228 - 229 (1998)
An improved synthesis of the antiulcer drug ranitidine from N-[2-[[[5- (hydroxymethyl)-2-furanyl]-methyl]-thiol]-ethyl]-N'methyl-2-nitro-1,1- ethenediamine is reported.
Ranitidine oral preparation for treating new indications of erosive esophagitis
-
Paragraph 0240; 0241; 0242; 0243; 0244-0247; 0274; 0287, (2018/11/22)
The invention discloses a ranitidine and a preparation method thereof, as well as a ranitidine preparation, a compound preparation and a preparation method thereof. The ranitidine has a low impurity content, high stability, and the preparation method is simple; the ranitidine preparation and the compound preparation prepared therefrom have high bioavailability and safety. The ranitidine, the ranitidine preparation and the compound preparation provided by the invention can effectively treat and maintain the treatment of erosive esophagitis.
New method for synthesizing ranitidine
-
Paragraph 0034; 0039-0044; 0047; 0052-0057; 0059; 0064-0069, (2018/12/14)
The invention discloses a new method for synthesizing ranitidine. The method comprises the steps of synthesizing vinylidene chloride, synthesizing 1, 1-dichloro-2-nitroethylene, carrying out a ring-closing reaction, carrying out a ring-opening reaction in presence of a desiccant, and synthesizing the ranitidine. The method adopts an anhydrous environment in the preparation process of a ring-opening product, thus avoiding the interference with the reaction and the generation of impurities due to the presence of water, reducing the post-treatment work and increasing the utilization rate of the raw materials. The preparation method provided by the invention effectively increases the reaction yield of the ring-opening product, improves the purity of the ring-opening reaction, and reduces the reaction time; therefore, the yield and purity of the product ranitidine are improved, the production cost is lowered, and the method is more beneficial to industrial production.
Novel synthesis method for high-stability ranitidine bismuth citrate
-
Paragraph 0016, (2018/01/12)
The invention discloses a novel synthesis method for high-stability ranitidine bismuth citrate. The method includes the steps of: 1) preparing a raw material solution; 2) performing a reaction to prepare ranitidine; 3) purifying the ranitidine; 4) performing a salt forming reaction; 5) discolorizing and sterilizing a product; and 6) producing a ranitidine bismuth citrate finish product. The novel synthesis method, through reasonable process design, can increase the quality and stability of ranitidine, thereby improving the pharmacologic property and stability of the ranitidine bismuth citrate. The novel synthesis method is low in raw material cost, has gentle process conditions, is good in controllability and yield, and is suitable for industrial production.
Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis
Galkin, Konstantin I.,Krivodaeva, Elena A.,Romashov, Leonid V.,Zalesskiy, Sergey S.,Kachala, Vadim V.,Burykina, Julia V.,Ananikov, Valentine P.
, p. 8338 - 8342 (2016/07/19)
Spectral studies revealed the presence of a specific arrangement of 5-hydroxymethylfurfural (5-HMF) molecules in solution as a result of a hydrogen–bonding network, and this arrangement readily facilitates the aging of 5-HMF. Deterioration of the quality of this platform chemical limits its practical applications, especially in synthesis/pharma areas. The model drug Ranitidine (Zantac) was synthesized with only 15 % yield starting from 5-HMF which was isolated and stored as an oil after a biomass conversion process. In contrast, a much higher yield of 65 % was obtained by using 5-HMF isolated in crystalline state from an optimized biomass conversion process. The molecular mechanisms responsible for 5-HMF decomposition in solution were established by NMR and ESI-MS studies. A highly selective synthesis of a 5-HMF derivative from glucose was achieved using a protecting group at O(6) position.