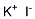Potassium iodide literature
Determination of micro-iodate in iodized salt by high performance liquid chromatography
Chen, Meilan,Ye, Mingli,Yang, Xinlei,Fawzi, El-Sepaia
, p. 7683 - 7686 (2014)
A novel method was developed to determine micro-iodate in iodized salt by high performance liquid chromatography via hydrazine hydrate reduction. Iodate was first reduced to iodide using 0.085 % hydrazine hydrate solution followed by HPLC analysis. The sample pretreatment process was simple without any significant interference from the large amount of chloride in the matrix sample during the final analysis. Linearity was validated in the range of 1 to 1000 μmol/L with high correlation coefficient (R2 = 0.9995) and the limit of detection (LOD) was 0.214 mg/kg (KIO3 content, S/N = 3). The relative standard deviation was less than 2 % (n = 5) and the spiked recoveries of four real samples were between 98.1 and 100.8 %. The proposed method was applicable for effective routine analysis of micro-iodate in iodized salt.
Extremely bulky copper(i) complexes of [HB(3,5-{1-naphthyl}2pz)3]- and [HB(3,5-{2-naphthyl}2pz)3]- and their self-assembly on graphene
Van Dijkman, Thomas F.,De Bruijn, Hans M.,Brevé, Tobias G.,Van Meijeren, Bob,Siegler, Maxime A.,Bouwman, Elisabeth
, p. 6433 - 6446 (2017)
The synthesis and characterization, using NMR (1H and 13C), infrared spectroscopy, and X-ray crystallography, of the ethene and carbon monoxide copper(i) complexes of hydridotris(3,5-diphenylpyrazol-1-yl)borate ([Tp(Ph)2]-) and the two new ligands hydridotris(3,5-bis(1-naphthyl)pyrazol-1-yl)borate ([Tp((1Nt))2]-) and hydridotris(3,5-bis-(2-naphthyl)pyrazol-1-yl)borate ([Tp((2Nt))2]-) are described. X-ray crystal structures are presented of [Cu(Tp(Ph)2)(C2H4)] and [Cu(Tp((2Nt))2)(C2H4)]. The compound [Cu(TpPh)2)(C2H4)] features interactions between the protons of the ethene ligand and the π-electron clouds of the phenyl substituents that make up the binding pocket surrounding the copper(i) center. These dipolar interactions result in strongly upfield shifted signals of the ethene protons in 1H-NMR. [Cu(Tp((1Nt))2)(CO)] and [Cu(Tp((2Nt))2)(CO)] were examined using infrared spectroscopy and were found to have CO stretching vibrations at 2076 and 2080 cm-1 respectively. The copper(i) carbonyl complexes form self-assembled monolayers when drop cast onto HOPG and thin multilayers of a few nanometers thickness when dip coated onto graphene. General macroscopic trends such as the different tendencies to crystallize observed in the complexes of the two naphthyl-substituted ligands appear to extend well to the nanoscale where a well-organized monolayer could be observed of [Cu(Tp((2Nt))2)(CO)].
Crystal structures and ionic conductivities of ternary derivatives of the silver and copper monohalides: I. Superionic phases of stoichiometry MA4I5: RbAg4I5, KAg4I5, and KCu4I5
Hull,Keen,Sivia,Berastegui
, p. 363 - 371 (2002)
The superionic properties of the compounds RbAg4I5, KAg4I5 and KCu4I5 have been investigated by powder neutron diffraction and complex impedance spectroscopy. RbAg4I5 and KAg4I5 have room-temperature ionic conductivities of σ = 0.21(6) and 0.08(5) Ω-1 cm-1, respectively, which increase gradually on increasing temperature. KCu4I5 is only stable in the temperature range between 515(5) K and its melting point of 605 K, and its ionic conductivity is σ = 0.61(8) Ω-1 cm-1, at T = 540 K. At lower temperatures, KCu4I5 disproportionates into KI + 4CuI and the ionic conductivity falls by over three orders of magnitude. Least-squares refinements of the powder neutron diffraction data for RbAg4I5 at ambient temperature confirm the reported structure (space group P4132, Z = 4, a = 11.23934(3) A), though with some differences in the preferred locations of the mobile Ag+. KAg4I5 and KCu4I5 are found to adopt the same basic structure as RbAg4I5, with the I- forming a β-Mn-type sublattice, with the K+ located in a distorted octahedral environment and the Ag+(Cu+) predominantly distributed over two sites which are tetrahedrally co-ordinated to I-. The implications for the conduction mechanism within these compounds are discussed, using a novel maximum entropy difference Fourier technique to map the distribution of the Ag+(Cu+) within the unit cell.
A versatile lead iodide particle synthesis and film surface analysis for optoelectronics
Awol, Nasir,Amente, Chernet,Verma, Gaurav,Kim, Jung Yong
, (2020)
Lead (II) iodide, PbI2, semiconductor was synthesized using versatile methods such as hydrothermal, refluxing, solid-state reaction, and co-precipitation for optoelectronics. All the PbI2 particles exhibited hexagonal-layered 2H structure in which the average crystallite size and optical bandgap (Eg) were 57 ± 10 nm and 2.31 eV, respectively. Then, PbI2films were prepared using dimethyl sulfoxide (DMSO) or dimethylformamide (DMF) on the top of a mesoporous TiO2 layer, yielding a wider Eg of 2.33–2.36. Finally, through water contact angle measurement, the surface and interfacial properties of the thin film were characterized, exhibiting initial solid-vapor surface tension (γsv) of 6.1–6.4 mJ/m2 and solubility parameter (δ) of 4.51–4.62 (cal/cm3)1/2. However, when these PbI2 films were exposed to H2O molecules in air, δ changes from 4.62 to 7.28 (cal/cm3)1/2 for PbI2 (DMSO) film or 4.51 to 12.98 (cal/cm3)1/2 for the PbI2 (DMF) film, respectively. Finally, by employing the theory of melting point depression combined with the Flory-Huggins lattice theory, the interfacial interactions between PbI2 and regioregular poly (3-hexylthiophene-2,5-diyl) were qualitatively characterized. The smaller the χ interaction parameter, the more depressed the melting point.
Thermal decomposition kinetics of potassium iodate
Muraleedharan,Kannan,Ganga Devi
, p. 943 - 955 (2011)
The thermal decomposition of potassium iodate (KIO3) has been studied by both non-isothermal and isothermal thermogravimetry (TG). The non-isothermal simultaneous TG-differential thermal analysis (DTA) of the thermal decomposition of KIO3<
Ion Exchange of Layered Alkali Titanates (Na2Ti3O7, K2Ti4O9, and Cs2Ti5O11) with Alkali Halides by the Solid-State Reactions at Room Temperature
Ogawa, Makoto,Saothayanun, Taya Ko,Sirinakorn, Thipwipa Tip
, p. 4024 - 4029 (2020/04/08)
Ion exchange of layered alkali titanates (Na2Ti3O7, K2Ti4O9, and Cs2Ti5O11) with several alkali metal halides surprisingly proceeded in the solid-state at room temperature. The reaction was governed by thermodynamic parameters and was completed within a shorter time when the titanates with a smaller particle size were employed. On the other hand, the required time for the ion exchange was shorter in the cases of Cs2Ti5O11 than those of K2Ti4O9 irrespective of the particle size of the titanates, suggesting faster diffusion of the interlayer cation in the titanate with lower layer charge density.