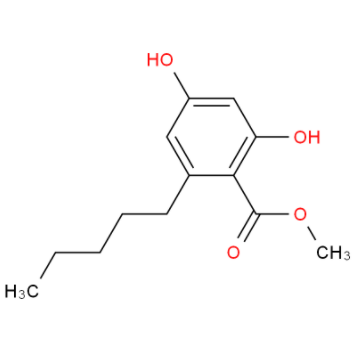
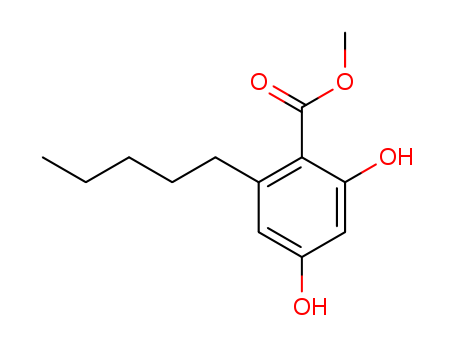
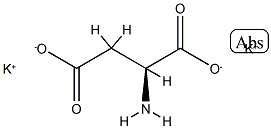
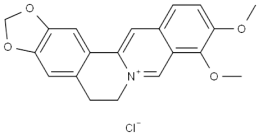
methyl 2,4-dihydroxy-6-pentylbenzoate literature
SYNTHESIS OF PHYTOCANNABINOIDS INCLUDING A DECARBOXYLATION STEP
-
Page/Page column 16, (2019/03/05)
method for decarboxylating a carboxylated phytocannabinoid compound of Formula I to form a phytocannabinoid compound of Formula II: Formula I Formula II wherein: R1 is selected from the group consisting of: substituted or unsubstituted C1-C5 alkyl; R2 is selected from the group consisting of: OH or O, and R3 is selected from the group consisting of: a substituted or unsubstituted cyclohexene, a substituted or unsubstituted C2-C8 alkene, or a substituted or unsubstituted C2-C8 dialkene; or R2 is O, and R2 and R3 together form a ring structure in which R2 is an internal ring atom; wherein the method includes heating a reaction mixture comprising the carboxylated phytocannabinoid compound and a polar aprotic solvent in the presence of a LiCl for a time sufficient to decarboxylate at least a portion of the carboxylated phytocannabinoid compounds and form the phytocannabinoid compound.
SYNTHESIS OF PHYTOCANNABINOIDS INCLUDING A DEMETHYLATION STEP
-
Page/Page column 16-17, (2019/03/05)
A method for demethylating a methylated phytocannabinoid compound of Formula I to form a phytocannabinoid compound of Formula II: Formula I Formula II wherein: R1 is selected from the group consisting of: substituted or unsubstituted C1-C5 alkyl; R2 is selected from the group consisting of: OH or O, and R3 is selected from the group consisting of: a substituted or unsubstituted cyclohexene, a substituted or unsubstituted C2-C8 alkene, or a substituted or unsubstituted C2-C8 dialkene; or R2 is O, and R2 and R3 together form a ring structure in which R2 is an internal ring atom; wherein the method includes: heating a reaction mixture comprising the methylated phytocannabinoid compounds and a polar aprotic solvent in the presence of a dissolved inorganic alkaline salt for a time sufficient to demethylate at least a portion of the methylated phytocannabinoid compounds and form the phytocannabinoid compound.
Chemistry of 1,3,5-Tris(trimethylsiloxy)-1-methoxyhexa-1,3,5-triene, a β-Tricarbonyl Trianion Equivalent
Chan, T. H.,Stoessel, D.
, p. 2423 - 2428 (2007/10/02)
The title compound has been synthesized and its chemistry studied.Condensation with orthoesters, acid chlorides, or imidazolides gave aromatic compounds in a 5C + 1C condensation.A formal synthesis of lasiodiplodin has been completed.
A BIOMIMETIC SYNTHESIS OF 1Δ-TETRAHYDROCANNABIOL
Chan, T. H.,Chaly, T.
, p. 2935 - 2938 (2007/10/02)
Condensation of 1,3-bis(trimethylsiloxy)-1-methoxybutadiene with the acid chloride 12 gave methyl olivetolate (13).Condensation of 13 with (+)-p-mentha-2,8-dien-1-ol gave methyl 1Δ-tetrahydrocannabiolate (14) in 55percent isolated yield.Alkaline hydrolysis of 14 gave 1Δ-tetrahydrocannabinol (1, 1Δ-THC).The synthesis is patterned after the biogenesis of 1.