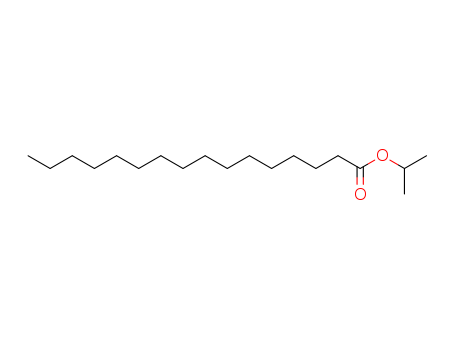
Isopropyl palmitate literature
Mammalian exocrine secretions. XIV: Constituents of preorbital secretion of steenbok, Raphicerus campestris
Burger,Greyling,Spies
, p. 2099 - 2108 (1999)
In a study aimed primarily at qualitative comparison of the organic constituents of the preorbital secretion of the steenbok, Raphicerus campestris, with those previously found in the preorbital secretion of the grysbok, R. melanotis, 109 compounds were identified in the secretion of the steenbok. Although the secretions from the two antelope are similar in that they are mostly long-chain, unbranched, saturated and unsaturated alcohols and various derivatives of these alcohols, only 22 of the identified compounds are present in both secretions. This is a small percentage of the more than 260 compounds present in the secretion of the steenbok, which is much more complex than that of the grysbok.
Mammalian exocrine secretions. XII: Constituents of interdigital secretions of bontebok, Damaliscus dorcas dorcas, and blesbok, D. d. phillipsi
Burger,Nell,Spies,Le Roux,Bigalke,Brand
, p. 2057 - 2084 (1999)
In addition to the nine compounds identified in the interdigital secretion of the bontebok, Damaliscus dorcas dorcas, in a previous study, 76 compounds belonging to different compound types, were identified in the interdigital secretions of the bontebok and the blesbok, D. d. phillipsi. These compounds include alkanes, alcohols, aldehydes, ketones, fatty acids, terpenoids, γ-lactones, an isopropyl ester, long-chain hydroxyesters, 2- substituted pyridines, phenols, steroids, and dimethylsulfone. No qualitative differences were found between secretions from the two sexes or from animals from different habitats. Although no attempt was made to correlate territorial behavior or other behavioral phenomena with the qualitative composition of interdigital secretions from individual animals, available information seems to indicate that quantitative differences probably do not have a major semiochemical function. Only two species of bacteria, Bacillus brevis and Planococcus citreus, were found in the interdigital pouches of male and female members of the two subspecies, regardless of the habitat of the animals. B. brevis synthesized, among other unidentified constituents, (Z)-3-penten-2-ol, 2-hexanone, 2-octanone, 2-nonanone, tetradecanoic acid, pentadecanoic acid, heptadecanoic acid, octadecanoic acid, (Z)-9-hexadecenoic acid, and isopropyl hexadecanoate in vitro, while P. citreus produced, among others, the γ-lactones dodecan-4-olide and (Z)-6-dodecen-4-olide, which is one of the major constituents of the interdigital secretions of both subspecies. Some components of the interdigital secretions are not present in the interdigital glandular tissue, and the possibility is discussed that these compounds could be produced by microbiological activity in the interdigital pouch.
Method for synthesis of long-chain fatty acid ester derivative
-
Paragraph 0029-0030, (2020/01/12)
The invention relates to a method for synthesis of a long-chain fatty acid ester derivative. Specifically, a hydrochloride of glycine methyl ester or glycine ethyl ester is used as a catalyst to catalyze the esterification reaction of long-chain fatty acid. The method includes: subjecting alcohol and long-chain fatty acid to esterification reaction under the action of the catalyst at certain temperature condition, then conducting extraction and precipitation with ethyl acetate, performing flushing with a sodium chloride aqueous solution for purification. A hydrochloride of glycine methyl esteror glycine ethyl ester is adopted as the catalyst, which belongs to a green catalyst, is the development trend of modern chemistry, has the characteristics of no corrosion to the reaction kettle, lowprice, no toxicity and the like, and is suitable for use as a catalyst to produce palmitate and laurate perfume raw materials.
Method for catalytically synthesizing isopropyl palmitate by using silica gel immobilized multi-sulfonic functionalized ionic liquid
-
Paragraph 0033-0047, (2019/01/06)
The invention discloses a method for catalytically synthesizing isopropyl palmitate by using a silica gel immobilized polysulfonic functionalized ionic liquid, and belongs to the technical field of synthesis of ester compounds in organic chemistry. The invention solves the technical problems of preparing isopropyl palmitate by using conventional methods such as an acyl chloride method, a direct esterification method and the like in the prior art, and overcomes the defects that the recovery rate of an ionic liquid is not high and the price of the ionic liquid is high in actual use. According tothe invention, palmitic acid and isopropyl alcohol are used as raw materials, a silica gel immobilized polysulfonic functionalized ionic liquid is used as a catalyst, and isopropyl palmitate is catalytically synthesized through direct esterification under a heating condition. The method has the advantages of simple process, mild conditions, simple and convenient post-treatment, low cost, and goodcatalytic effect; the obtained product has no color or low color and has high quality; the reaction esterification rate can reach 95-98%; and the catalysis activity is still good after the catalyst is recycled for multiple times.
USE OF ENDOCANNABINOID-LIKE COMPOUNDS FOR TREATING CNS DEGENERATIVE DISORDERS
-
Paragraph 0054-0056, (2017/09/12)
no abstract published
Preparation of phenol- or thiophenyl-sulfonic acid functionalized solid acids
-
Page/Page column 10; 11, (2015/07/02)
Some aryl sulfonic acid-functionalized solids were prepared by a new method. The catalytic activities of esterification by the prepared aryl sulfonic acid-functionalized solids were also tested.