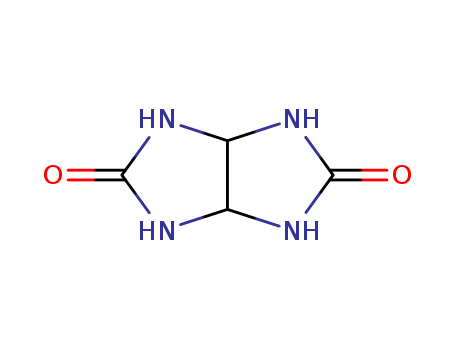
Glycoluril literature
Understanding behaviour of vitamin-C guest binding with the cucurbit[6]uril host
Pandey, Shubham,Soni, Vineet Kumar,Choudhary, Ganpat,Sharma, Pragati R.,Sharma, Rakesh K.
, p. 387 - 394 (2017)
The present study describes non-covalent interaction and complexation behaviour of sodium ascorbate (SA) with cucurbit[6]uril (CB[6]) at neutral pH in aqueous Na2SO4 solution. The interaction behaviour is investigated using various analytical techniques like NMR, UV–Vis, fluorescence, TGA and DRS. The substantial increase in the intensity of emission and absorption spectra of sodium ascorbate is observed. The Benesi–Hildebrand evaluation method is used to determine the stoichiometry and equilibrium constant of the cucurbit[6]uril–sodium ascorbate complex, which suggested the 1:1 complex. Time-dependent 1H NMR, 13C CP MAS and CD studies also echoed non-covalent interaction between SA with CB[6].
Effect of glycoluril and its derivatives on the flame resistance and physico-mechanical properties of rubber
Sal’keeva,Bakibaev,Khasenova,Taishibekova, Ye. K.,Sugralina,Minaeva, Ye. V.,Sal’keeva
, p. 132 - 139 (2016)
The effect of bicyclic bisureas (glycolurils) and their derivatives functionalized with hydroxymethyl, halomethyl, and dimethoxyphoshorylmethyl groups on the flame resistance and physico-chemical properties of synthetic isoprene and divinyl rubbers was studied, and the procedures for preparing these agents were suggested. The rubbers with the addition of the synthesized bicyclic bisureas demonstrated satisfactory levels of flame resistance and physico-mechanical parameters.
Efficient method for the cycloaminomethylation of glycoluril
Wingard, Leah A.,Johnson, Eric C.,Sabatini, Jesse J.
, p. 1681 - 1682 (2016)
The efficient method for the cycloaminomethylation of glycoluril to yield 2,6-ditert-butylhexahydro-1H,5H-2,3a,4a,6,7a,8a-hexaazacyclopenta[def]fluorene-4,8-dione is described. The material is synthesized employing water as the solvent, and is isolated by filtration. This is an improvement over the previous reported synthetic method, which relied on the use of samarium trichloride catalysis, as well as silica gel column chromatographic purification to obtain the target product.
Modulation of Bambusuril Anion Affinity in Water
Havel, Vaclav,Babiak, Michal,Sindelar, Vladimir
, p. 8963 - 8968 (2017)
Neutral and negatively charged anion receptors functioning in pure water are rare in supramolecular chemistry. Moreover, studies on adjusting the affinity of such receptors toward anions in water are absent from the literature. Two new bambusurils, 1 a and 2 a, were prepared to demonstrate that the affinity of bambusurils towards anions can be altered by the length of carboxyalkyl groups attached to the macrocycles. The stability of the bambusuril complexes was further controlled by the pH value. The crystal structure of bambusuril 1 a was described, in which two carboxyalkyl arms fold into the macrocycle cavity, thus forming the intramolecular self-inclusion complex.
Ring-opening hydrolysis of spiro-epoxyoxindoles using a reusable sulfonic acid functionalized nitrogen rich carbon catalyst
Patel, Parth,Tak, Raj Kumar,Parmar, Bhavesh,Dabas, Shilpa,Patel, Brijesh,Suresh, Eringathodi,Khan, Noor-Ul H.,Subramanian, Saravanan
, p. 12808 - 12814 (2021/04/14)
Controlling the product selectivity of a ring-opening hydrolysis reaction remains a great challenge with mineral acids and to an extent with homogeneous catalysts. In addition, even trace amounts of metal impurities in a bioactive product hinder the reaction progress. This has necessitated the development of robust and metal-free catalysts to offer an alternative sustainable route. We report a nitrogen-rich sulfonated carbon as a catalyst derived from an inexpensive precursor for the synthesis of bioactive vicinal diols of spiro-oxindole derivatives. The well-characterized catalyst shows wide generality with different electronic and steric substituents in the substrates under mild reaction conditions. Hot filtration test confirms no leaching of the acid moiety and the catalyst could be reused for four cycles with retention of activities.
Synthesis of Glycoluril using Urea Phosphate
Karpagalakshmi, K.,Lakshminarayanan, P.,Prakash, R.,Ramalakshmi, S.,Selvapalam, N.,Usha, G.,Yang, C.
, p. 1988 - 1992 (2022/01/24)
Abstract: Glycolurils are building blocks for the synthesis of cucurbiturils that are important host materials for several applications. Glycolurils are prepared conveniently by the condensation of 1,2-diketones with urea using an acid as catalyst. Herein, we report a facile method of synthesis of various glycolurils using urea phosphate and 1,2-diketones.
Critical Parameters for Green Glycoluril Synthesis
Araki, K.,Bertolucci, M. M.,Demets, G. J. F.,Oliveira, A. de,Soares, R. S. B.,Souza, L. R. R.
, p. 739 - 742 (2021/06/02)
Abstract: We have studied the critical parameters of Micheletti’s glycoluril synthesisstarting from urea and glyoxal on a statistical and multivariate analysis basis[12]. The process ispotentially highly useful for scaling-up glycoluril production, and needs to beoptimized. We have come to a conclusion thatH3PO4 could be used as well asP4O10 giving high yields attemperature around 95°C, using glyoxal without prior purification. All reactionshave been completed in less than 25 min with the yield as high as 80% under theoptimized conditions.
D - galactose bonded ring-opening cucurbituril and application thereof
-
Paragraph 0032, (2021/06/22)
The invention belongs to the field of supermolecular drug carriers and discloses D-galactose-bonded open-ringed cucurbituril with a structural formula shown in the specification, wherein R is a groupshown in the specification or a halogen, and at least one R is the group shown in the specification. The open-ringed cucurbituril containing a D-galactose group is a macrocyclic cryptand with an innercavity, and the open-ringed structure makes the ring rigidity of the macrocycle of the D-galactose-bonded open-ringed cucurbituril reduced and the D-galactose-bonded open-ringed cucurbituril has extremely strong host-guest bonding capacity; and the galactose-containing open-ringed cucurbituril disclosed by the invention has good water solubility, can be used as a drug carrier to be widely appliedto drug transportation, can also be used for greatly improving the bioavailability and stability of a drug and has potential tumor cell targeting property.