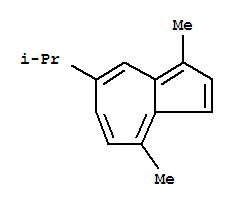
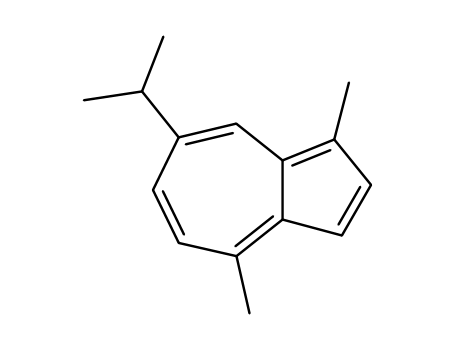
Guaiazulene literature
ALISMOL AND ALISMOXIDE, SESQUITERPENOIDS OF ALISMA RHIZOMES
Oshima, Yoshiteru,Iwakawa, Tsuneo,Hikino, Hiroshi
, p. 183 - 186 (1983)
From the crude drug 'takusha', wich is the rhizomes of Alisma plantago-aquatica var. orientale, two new sesquiterpenoids, alismol and alismoxide, have been isolated.Their structures have been estabished on the basis of chemical and physical evidence to be 1β,5β-guaia-6,10(15)-dien-4-ol and 4,10-epoxy-1β,5β-guaia-6-ene, respectively. - Key Word Index: Alisma plantago-aquatica var. orientale; Alismataceae; sesquiterpenoid; guaiane skeleton; alismol; 1β,5β-guaia-6,10(15)-dien-4-ol; alismoxide; 4,10-epoxy-1β,5β-guaia-6-ene
-
Ukita et al.
, p. 4584,4586 (1954)
-
Reaction of azulene with 1,2-bis[4-(dimethylamino)phenyl]-1,2-ethanediol in a mixed solvent of methanol and acetonitrile in the presence of hydrochloric acid: A facile one-pot synthesis and properties of new triarylethylenes possessing an azulen-1-yl group
Takekuma, Shin-Ichi,Kaibara, Masamichi,Minematsu, Toshie,Takekuma, Hideko
experimental part, p. 4780 - 4792 (2011/08/03)
Reaction of azulene (1) with 1,2-bis[4-(dimethylamino)phenyl]-1,2- ethanediol (2) in a mixed solvent of methanol and acetonitrile in the presence of 36% hydrochloric acid at 60 °C for 3 h gives 2-(azulen-1-yl)-1,1-bis[4- (dimethylamino)phenyl]ethylene (3) (8% yield), 1-(azulen-1-yl)-(E)-1,2-bis[4- (dimethylamino)phenyl]ethylene (4) (28% yield), and 1,3-bis{2,2-bis[4- (dimethylamino)phenyl]ethenyl}azulene (5) (9% yield). Besides the above products, this reaction affords 1,1-di(azulen-1-yl)-2,2-bis[4-(dimethylamino) phenyl]ethane (6) (15% yield), a meso form (1R,2S)-1,2-di(azulen-1-yl)-1,2- bis[4-(dimethylamino)phenyl]ethane (7) (6% yield), and the two enantiomeric forms (1R,2R)- and (1S,2S)-1,2-di(azulen-1-yl)-1,2-bis[4-(dimethylamino)phenyl] ethanes (8) (6% yield). Furthermore, addition reaction of 3 with 1 under the same reaction conditions as the above provides 6, in 46% yield, which upon oxidation with DDQ (=2,3-dichloro-5,6-dicyano-1,4-benzoquinone) in dichloromethane at 25 °C for 24 h yields 1,1-di(azulen-1-yl)-2,2-bis[4- (dimethylamino)phenyl]ethylene (9) in 48% yield. Interestingly, reaction of 1,1-bis[4-(dimethylamino)phenyl]-2-(3-guaiazulenyl)ethylene (11) with 1 in a mixed solvent of methanol and acetonitrile in the presence of 36% hydrochloric acid at 60 °C for 3 h gives guaiazulene (10) and 3, owing to the replacement of a guaiazulen-3-yl group by an azulen-1-yl group, in 91 and 46% yields together with 5 (19% yield) and 6 (13% yield). Similarly, reactions of 2-(3-guaiazulenyl)-1,1-bis(4-methoxyphenyl)ethylene (12) and 1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}-2-(3-guaiazulenyl)ethylene (13) with 1 under the same reaction conditions as the above provide 10, 2-(azulen-1-yl)-1,1-bis(4-methoxyphenyl)ethylene (16), and 1,3-bis[2,2-bis(4- methoxyphenyl)ethenyl]azulene (17) (93, 34, and 19% yields) from 12 and 10 and 2-(azulen-1-yl)-1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}ethylene (18) (97 and 58% yields) from 13.
Approach to the blues: A highly flexible route to the azulenes
Carret, Sebastien,Blanc, Aurelien,Coquerel, Yoann,Berthod, Mikael,Greene, Andrew E.,Depres, Jean-Pierre
, p. 5130 - 5133 (2007/10/03)
(Chemical Equation Presented) A palette of blues: Chlorobicyclo-[5.3.0] decatrienones are readily prepared from cycloheptatrienes by cycloaddition of dichloroketene, ring expansion with a diazoalkane, and dehydrochlorination in dimethylformamide. These compounds are used as intermediates in the regiocontrolled synthesis of a wide variety of natural and nonnatural azulenes (see scheme).
Chemistry of (+)-aromadendrene. Part 6: Rearrangement reactions of ledene, isoledene and their epoxides
Moreno-Dorado, F. Javier,Lamers, Yvonne M. A. W.,Mironov, Grigore,Wijnberg, Joannes B. P. A.,De Groot, Aede
, p. 7743 - 7750 (2007/10/03)
The chemistry of (+)-ledene and (-)-isoledene, both easily available from (+)-aromadendrene has been investigated. Reactions at the double bond of ledene take place preferably from the β-side. Under acidic conditions its C7-C8 β-epoxide and β-diol preferably react via carbocations, which are initially formed at C8. Rearrangement takes place to compounds with cubebane and cadinane skeletons. The reaction pattern of isoledene and its α-epoxide, under acidic conditions, is governed by the easy formation of an intermediate α-cyclopropylcarbinyl carbocation. Further reactions lead to products in which the C2-C3 bond of the cyclopropane ring is broken to give compounds with a guaiane skeleton. Guaiane-type dienes and unsaturated cyclic ethers are the final products of these rearrangements. Several derivatives of these compounds have been prepared.
Reaction of Guaiazulene with Bromine in Hexane and in Aqueous Tetrahydrofuran
Nozoe, Tetsuo,Shindo, Kimio,Wakabayashi, Hidetsugu,Kurihara, Teruo,Uzawa, Jun
, p. 687 - 688 (2007/10/02)
2-Bromoguaiazulenium bromide and 3,3-dibromo guaiazulenium bromide were obtained respectively from the reaction of guaiazulene and its 3-bromo compound with 1 equivalent of bromine in hexane at -20 deg C.The former compound afforded in methanol a mixture of guaiazulene, 3,3'-biguaiazulene, and oligomers, and gave 3-bromoguiazulene quantitatively with alkali.Dibromoguiazulenium bromide afforded with further moles of bromine in aqueous THF a mixture of guaiazulenequinone, 3-bromo-1-hydroxyquaiazulen-5-one, and a dark blue solid A in different ratios depending on the reaction conditions.